A large variation in the content of fibrous constituents
is found among species. The content of NDF
between the species varies from 18.5 to 38.7 % of DM (average 29.5 %). However,
a considerable variation of crude fiber (CF) content is found between varieties
of cassava leaves (CL) (i.e. 9.7 to 14.6 % of DM).
Green forages generally have an acceptable amino acid (AA) profile in comparison to e.g. alfalfa and soybean meal (Figure 1). The sum of essential AA (EAA) as percentage of CP varies between 38.6 and 48.9 %. According to Phuc et al (2000) (Table 2) the average lysine (Lys) content of the biomass products was 4.3 % of CP. An inter-species comparison revealed an inferior content of Lys in the CP of GF and Tric compared with other species. For threonine (Thr) the variation between species was limited, with an average of 4.1 % of CP. The contents of Thr in DW, Tric, Indicago, Mb, and CL were quite acceptable compared with the ideal AA pattern for pigs, while the concentration of the S-containing AA, Met and Cys (average 1.5 % of CP), would be limiting (NRC 1998). The AA composition of these forages is therefore of great importance when the feeds are used as protein sources for monogastric animal species.
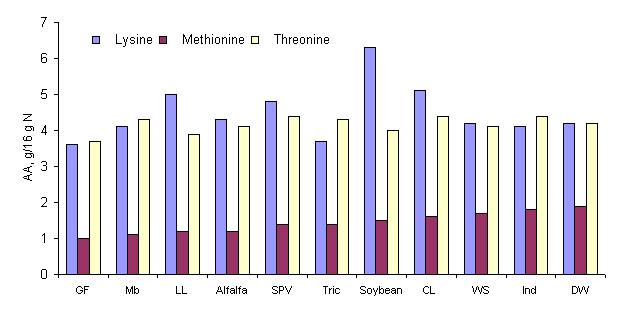
Figure 1. Amino acid contents (g/16 g N) of various forage
biomass products
Effect of inclusion of forage biomass on feed intake
Increased levels of protein replacement by forage biomass
CP in pig diets at the expense of soybean, leads to increased fibre contents of
the diets. The variation of inclusion level of biomass products has to be
addressed as an important factor for the acceptability of the diets by the
animals. Reductions of feed intake have been noted at high levels of inclusion
of forages, especially for LL, Tric, Ind and CL (Phuc et al 2001). This was
partly due to the increased content of dietary fibre but also possibly to
anti-nutritional factors present in the biomass products. Most soluble
polyphenolics have a bitter or astringent taste (Van Soest 1994; Kumar and
D'Mello 1995), which would also tend to reduce intakes. The bitter taste of LL
has been attributed partly to the presence of tannins as well as saponins
(D'Mello and Fraser 1981). High HCN levels in CL were also seen in the studies
by Lee and Hutagalung (1972) and Mahendranathan (1971).
Effect of inclusion of forage biomass on digestibility and
N-retention
The studies of Phuc and Lindberg (2000a) have clearly demonstrated that increasing levels of inclusion of biomass products in diets for growing pigs, lowered the ileal and total tract digestibility of OM and CP (Table 3), in accordance with earlier reports (Kornegay 1978; Kennelly and Aherne 1980; Sarwat et al. 1988). Figure 2 illustrates the relationship between the dietary NDF content and dOM for rats and pigs. This can be explained by differences in chemical composition between the basal diet and the biomass products, and the depressive effect of fibrous constituents on the apparent digestibility of the non-fibrous components of the diet (Fernandez and Jørgensen 1986).
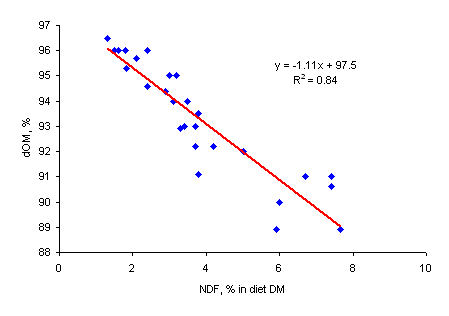 |
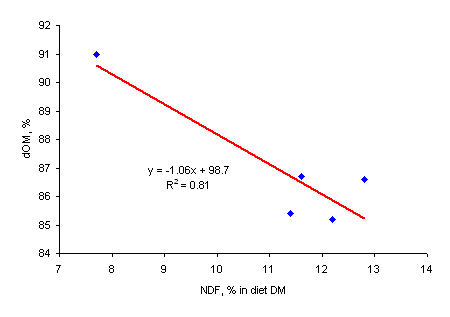 |
|
| Figure 2a. Relationship between organic matter digestibility (dOM, %) and dietary NDF content (% of DM) in rats | Figure 2b. Relationship between organic matter digestibility (dOM, %) and dietary NDF content (% of DM) in pigs |
The negative effects of
dietary fibre are partly a result of a reduced transit time of the ingesta in
the small intestine caused by fibrous components, limiting the time for nutrient
digestion and absorption (Low 1982; Den Hartog et al 1985). The extent of the
reduction in digestibility has been shown to vary with the level of fibre and
the feeding level (Dierick et al 1989; Ravindran 1990). Furthermore, the rate
of diffusion of solubilized nutrients towards the mucosal surface may be slowed
down, and solubilized nutrients may also be adsorbed by fibrous constituents,
due to water binding (Bergner 1982). In this context the observation that the
process of nutrient digestion is greatly influenced by differences in the
chemical and physical structures of fibrous components has to be considered
(Chen et al 1982; Agarwall and Chauhan 1989). Furthermore, anti-nutritional
factors in the biomass products may have interfered with the process of
digestion (Makkar 1993). Tannins are known to occur in cassava leaves (Ravindran
1993; Oke 1994) as well as in Tric and LL (Rosales et al 1993), which may
adversely affect dCP. In addition, part of the dietary CP disappears in the
hindgut and it is reasonable to assume that this is the result of microbial
fermentation and breakdown (Mason and Palmer 1973). A large variation in the
disappearance of the nutrients in the hindgut demonstrates that the extent of
this process differs among individual animals (Table 3).
|
Table 3. Ileal and total tract apparent digestibility coefficients of nutrients and energy in the experimental diets† (Phuc and Lindberg 2000a) |
|||||||
|
|
Control |
Cassava leaves |
Groundnut foliage |
Leucaena |
SEM |
P-value |
|
|
|
|
Sun-dried |
Ensiled |
leaves |
|
|
|
|
Ileal |
|
|
|
|
|
|
|
|
Organic matter |
0.84 a |
0.76 b |
0.77 b |
0.79 c |
0.77 b |
0.01 |
*** |
|
Crude protein |
0.73 a |
0.63 b |
0.64 b |
0.67 c |
0.64 b |
0.01 |
*** |
|
Crude fat |
0.57 |
0.47 |
0.52 |
0.52 |
0.56 |
0.03 |
|
|
NFE |
0.90 a |
0.85 b |
0.86 b |
0.86 b |
0.85 b |
0.01 |
*** |
|
Crude fibre |
0.14 a |
0.23 b |
0.25 b |
0.31 c |
0.19 a |
0.02 |
*** |
|
NDF |
0.29 a |
0. 28 a |
0.27 a |
0.39 b |
0.28 a |
0.02 |
* |
|
ADF |
0.32 a |
0.26 b |
0.27 b |
0.35 a |
0.27 b |
0.03 |
** |
|
Energy |
0.84 a |
0.76 b |
0.76 b |
0.79 c |
0.75 b |
0.01 |
*** |
|
|
|
|
|
|
|
|
|
|
Total tract |
|
|
|
|
|
|
|
|
Organic matter |
0.91 a |
0.85 b |
0.87 c |
0.87 c |
0.85 b |
0.01 |
*** |
|
Crude protein |
0.83 a |
0.73 b |
0.73 b |
0.75 c |
0.70 b |
0.02 |
*** |
|
Crude fat |
0.64 a |
0.52 b |
0.55 b |
0.57 b |
0.58 b |
0.02 |
** |
|
NFE |
0.97 a |
0.94 b |
0.94 b |
0.95 b |
0.94 b |
0.01 |
*** |
|
Crude fibre |
0.55 a |
0.52 b |
0.51 b |
0.52 b |
0.54 a |
0.02 |
*** |
|
NDF |
0.67 a |
0.52 b |
0.52 b |
0.63 a |
0.49 b |
0.02 |
*** |
|
ADF |
0.61 a |
0.43 b |
0.46 b |
0.56 c |
0.47 b |
0.02 |
*** |
|
Energy |
0.90 a |
0.83 b |
0.84 b |
0.85 c |
0.83 b |
0.01 |
** |
|
a,b,c,d
Means with different superscripts within rows are different (P<0.05) |
|||||||
Comparison of digestibility of tropical and temperate biomass products
The results of the studies of Phuc et al (2001) (Table 4) on tropical herbages show that the digestibility coefficients were lower than those of temperate herbage reported by Lindberg and Andersson (1998). For dOM a regression coefficient of -1.1 per percentage unit increase in NDF versus -0.8 was found by the latter authors. The reason for this is the differences in composition between the materials studied, in which the character of temperate and tropical biomass products play an important role. Tropical herbages mature more rapidly, and their protein content falls to very low levels, and fibre content increases with maturity. In the wet tropics the herbage available is usually fibrous but lush (i.e. high in water content), whereas in drier areas the mature herbage becomes desiccated. In both cases digestibility may be reduced. Another factor of nutritional importance is that tropical herbages have higher tensile strength than temperate plants due to their having more vascular bundles, thick-walled bundle sheaths and hence more lignin. Also the mesophyl cells are more densely packed than those in temperate forages. The consequence of this is lower digestibility of tropical compared with temperate forages. However, the difference is much less for tropical legumes compared to temperate legumes, because they are more similar anatomically (Van Soest 1994).
|
Table 4. Regression equations of the crude protein digestibility (dCP) and nitrogen utilization (Nu ; N retention as a percentage of N intake) and overall on protein replacement level (x, i.e. 0, 25 or 50 %) and organic matter digestibility coefficients (dOM) of the diets and overall on dietary NDF content ( % of DM) (Phuc et al 2001) |
|||||||||
|
|
WS |
LL |
DW |
GF |
Tric |
Ind |
Mb |
CL |
Overall |
|
dCP |
|
|
|
|
|
|
|
|
|
|
Intercept |
92.5 |
92.5 |
93.2 |
92.3 |
93.0 |
93.0 |
92.5 |
93.0 |
92.1 |
|
Slope |
-0.42 |
-0.42 |
-0.30 |
-0.36 |
-0.60 |
-0.28 |
-0.34 |
-0.32 |
-0.36 |
|
R2 |
0.98 |
0.98 |
0.99 |
0.98 |
0.96 |
0.98 |
0.99 |
0.98 |
0.80 |
|
Nu |
|
|
|
|
|
|
|
|
|
|
Intercept |
70.5 |
71.0 |
71.3 |
70.5 |
72.7 |
71.2 |
71.0 |
71.5 |
71.8 |
|
Slope |
-0.42 |
-0.52 |
-0.40 |
-0.50 |
-0.68 |
-0.62 |
-0.40 |
-0.30 |
-0.49 |
|
R2 |
0.98 |
0.98 |
0.99 |
0.99 |
0.97 |
0.98 |
0.99 |
0.98 |
0.77 |
|
dOM |
|
|
|
|
|
|
|
|
|
|
Intercept |
95.6 |
95.3 |
97.6 |
96.6 |
97 |
97.0 |
96.4 |
96.9 |
97.7 |
|
Slope |
-0.77 |
-0.80 |
-0.90 |
-0.94 |
-1.37 |
0.96 |
-0.91 |
-0.88 |
-1.10 |
|
R2 |
0.74 |
0.94 |
0.87 |
0.83 |
0.43 |
0.98 |
0.57 |
0.86 |
0.80 |
|
†
For abbreviations see footnote Table 2
|
|||||||||
Digestibility of different fibre constituents
Studies with pigs have shown that part of the dietary
fibre had been digested at the terminal ileum and that this slightly increased
with the incorporation of biomass products in the diets (Table 3). This suggests
that pigs are able to digest a substantial part of plant fibre pre-caecally, as
has been documented in reports on lucerne, red clover, white clover and
perennial ryegrass (Lindberg et al.1995; Andersson and Lindberg 1997a; 1997b),
coastal Bermuda grass (Dierick 1989), malt culms, dark grains and wheatings
(Zoiopoulos et al 1983) and wheat bran and sugar beet pulp (Graham et al
1986). Due to a solubilization of fibre in the upper gastrointestinal tract
(Graham et al. 1986), the ileal digestibility of NDF is generally higher than
that of crude fibre. The digestibility of ADF, containing more resistant
components, could also be expected to be much lower at both the ileal and faecal
level (Table 3).
Digestibility of AA at the terminal ileum
Most AA are digested to a greater extent than the CP
(Table 5). The reduction in EAA apparent ileal digestibility when biomass
products are included in the diet is due to an increase in the ileal flow of AA
with increasing fibre content in the diets (Phuc and Lindberg 2000b; Reverter
and Lindberg 1998; Reverter et al 1999). Furthermore, an increase of
endogenous AA secretions could also be expected (Boisen and Moughan 1996;
Jondreville et al 2000). The magnitude of this effect will depend on the
digestibility of the basal diet, the level and type of the fibre and the
contribution of the fibre source to the dietary amino acid supply (Sauer et al
1980; Lenis et al 1996). In addition to fibre level and quality, the presence
of tannins (Eggum and Christensen 1975; Göhl and Thomke 1976) and enzyme
inhibitors (Kidder and Manner 1978) might also influence the digestibility of
protein and AA.
However, most AA are digested to a greater extent than CP, which
may be due to the effect of NPN content and other factors in the products. The
apparent ileal digestibilities of dietary Arg, His and Lys have been found to be
highest, while that of Thr was the lowest (Phuc et al 2000b). There was a
significant reduction (P<0.05) found in the apparent ileal digestibility of AA
(dAA) in diets with inclusion of CL and LL compared to a control diet (Phuc et
al 2000b) (Table 5).
|
Table 5. Apparent ileal digestibility of crude protein and of essential and non-essential amino acids in experimental diets † (Phuc and Lindberg 2000b) |
||||||
|
|
Diet |
|
||||
|
|
Control |
CLM |
CLE |
GF |
LLM |
SEM |
|
Crude protein |
0.73a |
0.63b |
0.64b |
0.72a |
0.65b |
0.01 |
Essential amino acids |
|
|
|
|
|
|
|
Arginine |
0.90a |
0.80b |
0.82 b |
0.87 a |
0.78 b |
.007 |
|
Histidine |
0.81 |
0.77 |
0.79 |
0.80 |
0.78 |
.017 |
|
Isoleucine |
0.79 a |
0.70 b |
0.69 b |
0.77 a |
0.71 b |
.007 |
|
Leucine |
0.84 a |
0.74 b |
0.76 b |
0.82 a |
0.75 b |
.005 |
|
Lysine |
0.85 a |
0.79 b |
0.79 b |
0.82 a |
0.77 b |
.007 |
|
Methionine |
0.77 a |
0.70 b |
0.71 b |
0.75 a |
0.73 b |
.009 |
|
Phenylalanine |
0.87 a |
0.76 b |
0.78 b |
0.84 a |
0.78 b |
.009 |
|
Threonine |
0.73 a |
0.66 b |
0.68 b |
0.72 a |
0.66 b |
.011 |
|
Tyrosine |
0.80 a |
0.75 b |
0.74 b |
0.77 a |
0.73 b |
.008 |
|
Valine |
0.75 a |
0.67 b |
0.66 b |
0.74 a |
0.67 b |
.008 |
|
Non-essential amino acids |
|
|
|
|
|
|
|
Alanine |
0.77 a |
0.70 b |
0.72 b |
0.76 a |
0.69 b |
.005 |
|
Aspartic acid |
0.83 a |
0.73 b |
0.75 b |
0.81 a |
0.75 b |
.006 |
|
Glutamic acid |
0.86 a |
0.79 b |
0.80 b |
0.83 a |
0.80 b |
.006 |
|
Glycine |
0.78 a |
0.70 b |
0.71 b |
0.76 a |
0.69 b |
.012 |
|
Proline |
0.82 |
0.77 |
0.76 |
0.80 |
0.77 |
.023 |
|
Serine |
0.81 a |
0.73 b |
0.75 b |
0.80 a |
0.72 b |
.008 |
|
† CLM = cassava leaf meal; CLE = ensiled
cassava leaves; GF = groundnut foliage; LLM = leucaena leaves |
||||||
Effect of preservation on chemical composition
In some studies sun-drying has been found to have only a
small effect on the CP content compared to 60
oC oven- drying of cassava leaf meal (CLM) (Phuc et al 2001b).
However, after drying at 105 oC, Maillard reactions occur and Lys
content has been found to decrease, most likely due to the formation of
lignin-like polymers, which also resulted in a higher fibre content (Van Soest
and Mason 1991). Ensiling has been found to have only a limited effect on
the content of EAA as compared with drying (Phuc et al 2001) (Table 6). The
high content of lactic acid and low content of ammonia as a percentage of total
N confirmed the ideal ensiling conditions (Van Soest 1994) resulting from cane
molasses addition (Table 7).
Table 6. Chemical composition (% of DM), and essential amino acid (EAA) and non-essential amino acid (NEAA) content (g per 16 g N) in the biomass products investigated (Phuc et al 2001b) |
|||||||
|
|
Cassava leaves |
Cassava leaves |
Sweet potato vines |
||||
|
|
Batch A |
|
Batch B |
|
|
||
|
|
Sun dried |
Dried 60 oC |
Dried 105 oC |
Sun dried |
Ensiled |
Sun dried |
Ensiled |
Chemical composition |
|
|
|
|
|
|
|
|
Organic matter |
92.8 |
92.4 |
92.8 |
91.4 |
92.8 |
89.9 |
86.6 |
|
Crude protein |
32.4 |
32.7 |
32.2 |
33.3 |
31.7 |
20.6 |
20.1 |
|
Ether extract |
6.4 |
7.5 |
8.2 |
7.2 |
8.1 |
2.5 |
3.3 |
|
NDF |
27.5 |
25.3 |
37.6 |
24.4 |
24.8 |
28.4 |
29.2 |
Essential amino acids |
|
|
|
|
|
|
|
|
Arginine |
6.3 |
6.4 |
5.8 |
6.5 |
5.6 |
6.0 |
5.9 |
|
Histidine |
2.2 |
2.0 |
2.0 |
1.8 |
1.7 |
2.0 |
2.2 |
|
Isoleucine |
4.1 |
4.4 |
4.5 |
4.2 |
4.2 |
4.2 |
4.2 |
|
Leucine |
8.7 |
8.9 |
9.1 |
8.3 |
8.3 |
8.2 |
8.0 |
|
Lysine |
5.1 |
5.1 |
4.2 |
5.5 |
5.4 |
4.8 |
4.9 |
|
Methionine |
1.6 |
1.4 |
1.5 |
1.6 |
1.4 |
1.4 |
1.2 |
|
Phenylalanine |
6.3 |
6.2 |
6.2 |
6.2 |
5.6 |
5.7 |
5.6 |
|
Threonine |
4.4 |
4.2 |
4.4 |
4.1 |
3.9 |
4.4 |
4.2 |
|
Tyrosine |
4.3 |
4.6 |
4.6 |
4.4 |
4.4 |
4.1 |
3.8 |
|
Valine |
5.9 |
5.6 |
5.7 |
5.6 |
5.3 |
5.4 |
5.5 |
|
S EAA |
48.9 |
48.8 |
48.0 |
48.2 |
45.8 |
46.2 |
45.5 |
|
S AA |
87.6 |
87.3 |
87.3 |
85.5 |
83.3 |
84.0 |
82.9 |
Table 7. pH and content of organic acids(% of DM) and ammonia (g per 100g N) inensiled cassava leaves (CLE) and ensiled sweet potato vines (SPV) |
||
|
|
CLE |
SPV |
|
pH |
3.8 |
4.0 |
|
Succinic acid |
0.4 |
0.6 |
|
Lactic acid |
8.5 |
9.6 |
|
Acetic acid |
1.0 |
1.0 |
|
Propionic acid |
0.2 |
- |
|
2,3 – butane-diol |
0.1 |
0.3 |
|
Butyric acid |
0.0 |
0.0 |
|
Ethanol |
1.7 |
5.3 |
|
Ammonia |
3.5 |
4.2 |
Anti-nutritional constituents
Anti-nutritional factors in livestock feedstuffs are
widespread. Consumption of foods containing these constituents may lower feed
intake, nutrient utilization, food conversion efficiency and hence animal
performance as well as economy. At high levels of dietary intake toxicity ensues
and sometimes even animals will die. An acute shortage of conventional
foodstuffs for the feeding of livestock in developing countries has forced
planners and nutritionists to look for unconventional feed resources, wherein
there is no competition with humans. These unconventional feed resources may
contain anti-nutritional constituents limiting their biological value.
Nevertheless, an unconventional food today could be a conventional food of the
future (Makkar 1993). Some of the biomass products under investigation have been
reported to contain anti-nutritional constituents such as tannins, cyanogenic
glucosides and mimosine.
Tannins
Most plant leaves contain tannins, which are a diverse
group of polyphenolic substances. Tannins can be defined as any phenolic
compound of moderately high molecular weight containing sufficient phenolic
hydroxyls and other suitable groups to effectively form strong complexes with
proteins and other macromolecules (Van Soest et al 1987). According to these
authors conventional classification recognises two types of tannins, (i) the
condensed tannins, which are polymeric forms of flavonols, and (ii) the
hydrolysable tannins which are esters of sugar and polyhydroxyphenolic acids.
These compounds have adverse effects on growth and have the capability to lower
the protein digestibility and amino acid availability, either by forming
indigestible complexes with dietary proteins or by inactivation of proteolytic
enzymes (Kumar and Singh 1984). Most soluble polyphenolics have a bitter or
astringent taste (Van Soest 1994). Reduced feed intake may occur due to the
slowdown in the digestion of the feed or due to low palatibility (Kumar and
D'Mello 1995). Tannin contents are reported to increase with maturity and vary
between cultivars (Ravindran 1993), and in cassava leaves vary from 30 to 50
g/kg DM (Ravindran 1993). LL contain tannins, with levels ranging from 12 to 44
g/kg DM (D'Mello and Acamovic 1989, Rosales et al. 1993). Tric also has been
reported to contain around 50 g/kg DM tannins (Rosales et al 1993).
Cyanogenic glucosides
The cyanogenic glucosides are toxic to animals when
hydrocyanide acid (HCN) is generated (Van Soest 1994). The release of free HCN
is brought about by the action of either the endogenous enzyme linamarase in
damaged plant tissues or by β-glucosidases within the digestive tract of
animals. Animals can detoxify cyanide via several pathways, but primarily by
reaction with thiosulphate to form thiocyanate. This conversion represents a 200
fold reduction in toxicity. Thiocyanate, however, is a potent goitrogen and has
been implicated in the aetiology of goitre in animals (Langer 1966; Shihombing
et al 1971) and humans (Ekpechi 1973). In animals, while acute cases of cyanide
toxicity usually result in sudden death, less severe cases may lead to
gastrointestinal disorders and growth depression (Hill 1973).
Since the sulphur needed for the detoxification of
cyanide is obtained from dietary methionine, the presence of cyanogenic
glucosides could lead to a deficiency of this essential amino acid at poor or
marginal supply of methionine, resulting in reduced animal performance (Oke
1978). Bitterness associated with high cyanogenic glucoside content in cassava
has been reported in a number of studies (Lee and Hutagalung 1972;
Mahendranathan 1971; Sundaresan et al. 1987). Cyanide is the main
anti-nutritional factor in CL that reduces the nutritional quality of the leaf
meal. Generally, the cyanide content ranges from 200 to 800 mg HCN/kg fresh leaf
but values as low as 80 mg/kg (Wood 1965) and as high as over 4,000 mg/kg
(Ravindran and Ravindran 1988) have been reported. Per kg DM the practical range
of HCN content would correspond to between 800 to 3,200 mg. The glucoside
concentration in cassava leaves decreases with the age (Lutaladio 1984;
Ravindran and Ravindran 1988) and nutritional status of the plant, and is
increased by e.g. N-fertilization (De Bruilin 1973). The elimination of
cyanogens by heating will depend on the temperature, the stage of development of
plant, and the type of heat. Simple sun-drying or oven drying has been reported
to eliminate almost 90 % (Phuc et al 2000), and sun-drying reduces the cyanogen
content of CL more effectively than ensiling (Table 8) (Phuc et al 2000, 2001b)
because of the stability of the linamarase at low pH values (Oke 1994). Despite
its high content of HCN, documented cases of poisoning due to the ingestion of
CL are rare (Ravindran 1993).
|
Table 8. Contents of total HCN, intermediary products (cyanohydrine), free HCN and glucoside (linamarin) (mg/kg DM) of cassava leaves |
|||||
|
|
Cassava leaves A |
Cassava leaves B |
|||
|
|
Sun dried |
Dried 60 oC |
Dried 105 oC |
Sun dried |
Ensiled dried |
|
Total HCN |
59 |
86 |
28 |
255 |
250 |
|
Intermediary (cyanohydrine) |
13 |
47 |
1 |
152 |
215 |
|
Free HCN |
13 |
33 |
9 |
62 |
5 |
|
Glucoside (Linamarin) |
33 |
6 |
18 |
42 |
30 |
|
HCN
content of ensiled cassava leaves (not dried): total HCN: 762;
Cyanohydrine: 673; Free HCN: 17 and Linamarin: 73. |
|||||
Mimosine
Mimosine is an alkaloid [beta-N-(3-hydroxy-4-pyridone)]
found in Leucaena leucocephala (LL), a legume widely distributed in tropical
areas as a fodder tree. Mimosine has been shown to be responsible for some
animal disorders. Crounse et al (1962) suggested that mimosine may interfere
with tyrosine metabolism by preventing iodination of tyrosine, the first step in
the synthesis of thyroxine, resulting in goitre and loss of appetite. Mimosine
poisoning also causes loss of hair (Jones et al 1976) and poor reproductive
performance. The mimosine content of fresh LL can be decreased by heating to
temperatures > 70 oC, or by addition of iron salts (i.e.ferrous
sulphate) (National Academy of Sciences 1977, Meulen et al 1979; D'Mello and
Acamovic 1989; Kumar and D'Mello 1995; Laswai et al 1997). The mimosine content
can also be reduced by soaking in water and drying. Concentrations of mimosine
in the leaf range from 10 to 25 g/kg DM, with even higher quantities of up to
145 g/kg DM in the seeds (D'Mello 1991). For a time Australian agronomists
selected low mimosine strains in leucaena (Van Soest 1994). However, Brewbaker
and Hutton (1979), Chen et al. (1982) and Phuc and Lindberg (2000) observed no
harmful effects when including 10 % and 16 % LL in diets for growing pigs,
although Wayman et al (1970) showed that diets containing 15 % LL reduced the
ability of gilts to conceive and reduced average litter size and weight.
Saponins
Saponins are glycosides containing a polycyclic aglycone
moiety of either C27 steroid or C30 triterpenoid attached to the carbohydrate.
They are widely distributed in the plant kingdom and have a characteristic
bitter taste (Kumar and D'Mello 1995). According to Basu and Rastogi (1967) and
Oakenfull (1981) LL also contain saponins which have adverse effects on
monogastric animal growth and may also affect cholesterol metabolism (Oakenfull
1981; Cheeke 1976). The bitter taste of LL has been attributed partly to the
presence of tannins (D'Mello and Fraser 1981) and partly to saponins.
Other anti-nutritional factors
D'Mello and Acavomic (1989) claim that other
anti-nutritional factors, such as protease inhibitors and galactomannan gums,
are present in biomass products and may also reduce performance. There is
evidence of relative low in vitro digestion of NDF and organic matter in
Indicago hirsusta (Ind) (Brown and Pitman 1991) and early references (Bailey
1906; cited by Göhl 1981) refer to suspected poisoning of stock by Ind, but
these suspicions apparently have not been confirmed.
Estimations of the nutritive value of forages in pigs
The chemical composition of forage biomass products tested with pigs and their nutritive value estimated by the difference method and by the regression method are shown in Tables 9 and 10 (Phuc et al 2001a; Phuc and Lindberg 2000b).
|
Table 9. Estimated digestible energy (DE, MJ/kg DM), digestibility (%) of organic matter (dOM) and crude protein (dCP) of biomass products investigated † (Phuc et al., 2001b) |
||||||||
|
|
WS |
LL |
DW |
GF |
Tric |
Ind |
Mb |
CL |
|
DE ‡ |
10.8 |
9.6 |
8.2 |
7.8 |
5.1 |
12.7 |
8.6 |
11.5 |
|
dOM |
57 |
43 |
50 |
50 |
33 |
65 |
54 |
59 |
|
dCP |
51 |
46 |
64 |
57 |
34 |
64 |
59 |
61 |
|
†For abbreviations see footnote Table 1 |
||||||||
|
Table 10. Gross energy (GE, MJ per kg DM), and organic matter (OM), crude protein (CP), NDF content (% of DM) and estimated digestible energy (DE, MJ/kg DM), digestibility (%) of organic matter (dOM) and crude protein (dCP) of biomass products investigated in pigs (Phuc et al 2000a, 2001b) |
||||||||
|
|
|
LL |
GF |
CL |
ECL |
|||
Chemical composition |
|
|
|
|
|
|
||
|
GE |
|
21.5 |
18.1 |
19.2 |
21.4 |
- |
21.2 |
- |
|
OM |
|
91.4 |
90.6 |
91.4 |
91.3 |
89.1 |
89.5 |
89.7 |
|
CP |
|
28.3 |
17.5 |
33.3 |
26.4 |
26.0 |
24.5 |
27.6 |
|
NDF |
|
37.5 |
41.9 |
24.4 |
32.1 |
33.5 |
32.6 |
33.5 |
Digestibility |
|
|
|
|
|
|
|
|
|
DE |
|
10.9 |
10.8 |
11.3 |
11.0 |
- |
12.0 |
- |
|
dOM |
|
53 |
64 |
59 |
54 |
51 |
59 |
52 |
|
dCP |
|
42 |
47 |
59 |
45 |
44 |
46 |
59 |
The coefficients of digestibility of amino acids of some biomass products are shown in Table 11 (Phuc and Lindberg 2000b).
|
Table 11. Apparent ileal digestibility of crude protein, essential and non-essential amino acids in sun-dried cassava leaves, ensiled cassava leaves, groundnut foliage and leucaena leaves |
||||||||
|
|
Cassava leaves |
Groundnut |
Leucaena |
|||||
|
|
sun-dried |
ensiled |
foliage |
leaves |
||||
|
|
Mean |
SD |
Mean |
SD |
Mean |
SD |
Mean |
SD |
|
Crude protein |
0.37 |
0.02 |
0.37 |
0.03 |
0.43 |
0.07 |
0.39 |
0.05 |
|
Essential amino acids |
|
|
|
|
|
|
|
|
|
Arginine |
0.50a |
0.07 |
0.56 a |
0.05 |
0.77 b |
0.04 |
0.48 a |
0.03 |
|
Histidine |
0.61 |
0.05 |
0.68 |
0.03 |
0.73 |
0.04 |
0.67 |
0.05 |
|
Isoleucine |
0.48 a |
0.02 |
0.45 a |
0.04 |
0.71 b |
0.05 |
0.52 a |
0.05 |
|
Leucine |
0.50 a |
0.03 |
0.57 a |
0.02 |
0.72 b |
0.09 |
0.52 a |
0.03 |
|
Lysine |
0.64 a |
0.04 |
0.64 a |
0.04 |
0.73 b |
0.06 |
0.61 a |
0.07 |
|
Methionine |
0.56 a |
0.03 |
0.55 a |
0.04 |
0.73 b |
0.05 |
0.57 a |
0.12 |
|
Phenylalanine |
0.55 a |
0.05 |
0.52 a |
0.06 |
0.68 b |
0.07 |
0.55 a |
0.05 |
|
Threonine |
0.52 a |
0.07 |
0.54 a |
0.02 |
0.69 b |
0.05 |
0.52 a |
0.04 |
|
Tyrosine |
0.64 |
0.06 |
0.61 |
0.03 |
0.65 |
0.06 |
0.60 a |
0.06 |
|
Valine |
0.60 a |
0.05 |
0.62 a |
0.03 |
0.72 b |
0.04 |
0.61 a |
0.03 |
|
Non-essential amino acids |
|
|
|
|
|
|
||
|
Alanine |
0.56 a |
0.02 |
0.57 a |
0.04 |
0.73 b |
0.09 |
0.54 a |
0.02 |
|
Aspartic acid |
0.60 a |
0.04 |
0.62 a |
0.03 |
0.76 b |
0.06 |
0.60 a |
0.05 |
|
Glutamic acid |
0.55 a |
0.08 |
0.53 a |
0.04 |
0.67 b |
0.06 |
0.57 a |
0.07 |
|
Glycine |
0.50 a |
0.06 |
0.54 a |
0.08 |
0.69 b |
0.07 |
0.49 a |
0.09 |
|
Proline |
0.62 a |
0.04 |
0.57 a |
0.07 |
0.74 b |
0.05 |
0.63 a |
0.04 |
|
Serine |
0.62 a |
0.03 |
0.61 a |
0.04 |
0.78 b |
0.04 |
0.64 a |
0.03 |
|
a, b, c, d Means with different superscripts within rows are significantly different (P<0.05) |
||||||||
Effect of including forages in diets for growing pigs
It is necessary to select forages suitable for pigs, and
to determine the extent to which they can contribute to the supply of nutrients
for different categories of animal. This presupposes knowledge of the chemical
characteristics of the biomass products, and of their nutritional limitations,
especially anti-nutritional factors. If these are known then combinations of
locally available products may be suggested as suitable alternatives to provide
an adequate supply of both energy and protein to meet the animals' nutritional
requirements.
The differences in response to forages depend on the material that forages replace in the diet. For example, when CLM replaced rice bran in diets for growing pigs it gave higher daily weight gain, probably because of the slightly inferior composition of the rice bran compared with the CLM. However, an inverse effect was found when the CLM replaced a basal diet, which was of higher nutritive value than the CLM (Phuc et al 2003). This was also found in other studies on growing pigs (Fernandez and Jørgensen 1986).
A number of studies have shown that the inclusion level of CLM can be up to 12-15 %, and of ensiled cassava leaves up to 9-13% in the diet of fattening pigs without strong negative effects on growth rate.(Du Thanh Hang et al 1999; Ly 2002; Nguyen Thi Loc1997, 1999; Phuc et al 2003).
To use CLM for pigs efficiently, it is necessary to identify the diet. Up to 15 % of CLM can be used in the diet without any problem. However, the optimum level is usually from 4 to 10 %, depending on factors such as the basal diet, prices and the availability of the leaves (Table 12).
|
Table 12. Effects of level of inclusion of CLM (% of DM) on the performance of growing-finishing pigs (Phuc et al 2003) |
||||
|
Level of inclusion |
0 % |
4% |
8% |
12% |
|
Growth performance |
|
|
|
|
|
Initial weight |
23.1 |
23.1 |
23.1 |
23.1 |
|
Final weight |
84.1 |
87.2 |
84.7 |
86.7 |
|
Daily weight gain (g) |
545 |
572 |
548 |
570 |
|
Relative to control (%) |
100 |
105 |
100 |
104 |
|
FCR |
3.24 |
3.24 |
3.27 |
3.33 |
|
Relative to control (%) |
100 |
100 |
101 |
103 |
|
Carcass and meat quality |
|
|
|
|
|
No of slaughtered pigs |
2 |
2 |
2 |
2 |
|
Live weight (kg) |
92.0 |
87.5 |
93.5 |
89.0 |
|
Carcass weight |
71.3 |
72.0 |
73.5 |
73.5 |
|
Percentage (%) |
77.5 |
82.3 |
78.6 |
82.6 |
|
Back fat (mm) |
17.6 |
15.5 |
8.0 |
7.5 |
|
Chemical composition of meat (%) |
|
|
|
|
|
Protein |
21.9 |
23.3 |
22.6 |
22.6 |
|
Lipid |
5.65 |
3.35 |
3.7 |
3.09 |
Effect of including forages in diets for pregnant sows
Pregnant sows can utilize high fiber diets, and so forage can be a useful feed ingredient for sows during pregnancy. It is not only high in protein but also in vitamins and minerals, and can give good reproductive performance. The limited energy requirement of pregnant sows means that sows can tolerate high fibre diets (up to 10% of fibre). An experiment where 10%, 20% and 30% of CLM replaced rice bran (Phuc 20003) showed that the number of piglets born was higher in the groups fed CLM than in the control group (Table13), and due to greater litter size, the total weaning weights per litter were higher in the CLM fed groups.
The better performance of the CLM fed groups was possibly due to the higher content of vitamins and minerals and the better amino acid profile of CLM than of rice bran. Especially beta-carotene can increase the production of uterine specific proteins which support embryo survival. It also contains a basic glycoprotein with iron-binding capacity and a group of acidic proteins with immuno-suppressive capabilities. Beta-carotene also increases the production of progesterone during the initial formation of the corpora lutea.
|
Table 13. Effects of level of inclusion of CLM in the diet on the performances of pregnant sows (Phuc et al 2003) |
||||
|
|
0% |
10% |
20% |
30% |
|
Number of sows |
10 |
9 |
10 |
10 |
|
No.of piglets born/litter |
9.9 |
12.7 |
11.2 |
11.7 |
|
Difference to control (pigs) |
|
+2.8 |
+1.3 |
+1.8 |
|
No. of weaned pigs/litter |
8.5 |
10.1 |
9.2 |
9.3 |
|
Difference to control (pigs) |
|
+ 1.6 |
+ 0.68 |
+0.83 |
|
Birth weight (kg/litter) |
15.5 |
17.6 |
16.9 |
16.6 |
|
Birth weight (kg/piglet) |
1.6 |
1.4 |
1.7 |
1.4 |
|
Weaning weight/litter (kg) |
54.0 |
61.6 |
56.8 |
61.4 |
|
Difference to control (kg/litter) |
|
+ 7.6 |
+ 2.8 |
+7.4 |
|
Weaning weight (kg/piglet) |
6.3 |
6.1 |
6.2 |
6.6 |
|
Diarrhea rate (%) |
12.4 |
9.3 |
11.2 |
11.5 |
|
Feed intake/day/sow (kg) |
2.3 |
2.3 |
2.3 |
2.3 |
|
Feed intake/day/sow in lactation (kg) |
4.9 |
5.0 |
5.0 |
5.1 |
There were no indications of cyanide toxicity on any of the diets in the studies. It can be concluded that sun drying is a good processing method for eliminating the deleterious effects of HCN and to make CLM safe for animals. This result is in accordance with results reported by Phuc et al (2000; 2000a; 2001a, 2001b).
Conclusions
-
Marked differences in nutritive properties have been found in tropical biomass products that are potentially useful as feed resources for pigs, especially in their content of protein. However, methionine deficiency in many tropical forages may impose nutritional limitations.
-
Fiber content is known to be an important factor determining organic matter digestibility in different forages. The importance of harvesting at an early stage of development is more critical for pigs.
-
Sun-drying and drying at 60 oC, as well as ensiling, have been found to be suitable techniques for preserving green forages under tropical conditions.
-
Forages can be a source of benefit to pig farmers, especially if replacing a high fiber feed such as rice bran or coconut meal. Farmers have to make a decision as to what should be replaced. The determination of optimum levels of inclusion in pig diets, and also the effects of constraints such as anti-nutritional factors, need to be further investigated.
References
Agarwall V and Chauhan B M 1989: Effect of feeding some plant foods as source of dietary fibre on biological utilisation of diet in rats. Plant Foods in Human Nutrition, 39: 161-167.
Andersson C and Lindberg J E 1997: Forages in diets for growing pigs. 2. Nutrient apparent digestibilities and partition of nutrient digestion in barley-based diets including red-clover and perennial ryegrass meal. Animal Science, 65: 493-500.
Bergner H 1982: Fiber and nitrogen excretion. In: Physiologie digestive chez le porc, (ed. J.P. Laplace, T. Corring and A. Rerat), Les Colloques de l'INRA Jouy-en-Josas, Versailles, France. pp. 237-240.
Boisen S and Moughan P J 1996: Different expressions of dietary protein and amino acid digestibility in pig feeds and their application in protein evaluation: A theoretical approach. Acta Agriculturœ Scandinavica, Section A, Anim. Sci. 46: 165-172.
Brown W F and Pitman W D 1991:Concentration and degradation
of nitrogen and fibre fractions in selected tropical grasses and legumes.
Tropical Grasslands 25: 305-312.
Close W H 1993: Fibrous diets for pigs. Animal Production in Developing Countries, 16: 107-117.
Crounse R G, Maxwell J D and Blank H 1962: Inhibition of growth of hair by mimosine. Nature 194: 694.
D'Mello J P F 1991:Toxic amino acids. In: Toxic substances in Crop
Plants. (eds). D'Mello, J.P.F., Duffus, C.M. and Duffus, J.H. Royal Society
of Chemistry, Cambridge, pp. 21-48.
D'Mello J P F 1995: Leguminous leaf meals in non-ruminant nutrition. In: Tropical legumes in animal nutrition. (Eds) D'Mello, J.P.F and Devendra, P. Biddles, Guildford, London. pp 247-281.
D'Mello J P F and Acamovic T 1989: Leucaena leucocephala in Poultry Nutrition-A Review. Animal Feed Science and Technology, 26: 1-28.
D'Mello J P F and Fraser K W 1981: The composition of leaf meal from
Leucaena leucocephala, Tropical Science, 23: 75-78.
De Bruilin G H 1973: The cyanogenic character of cassava. In Chronic cassava toxicity. Proceedings of the International workshop, London. pp. 43-48.
Den Hartog L A, Verstegen M W A, Huisman J and van Kempen G J M 1985: Effect of dilution of a pig diet on the digestibility of the nutrients. Proceedings of 36th annual meeting of the European Association for Animal Production (EAAP). Halkidiki, Greece.
Dierick N A, Vervaeke I J, Demeyer D I and Decuypere J A 1989: An approach to the energetic importance of fibre digestion in pigs. I. Importance of fermentation in the overall energy supply. Animal Feed Science and Technology, 23: 141-167.
Dudley D and Culley J 1978: The use of Duckweed. American Scientist, 66: 442 - 451.
Fernández J A and Jørgersen N 1986: Digestibility and absorption of nutrients as affected by fiber content in the diet of the pig. Quantitative aspects. Livestock Production Science, 15: 53-71.
Garcia G W, Ferguson T U, Neckles F A and Archibald K A E 1996:The
nutritive value and forage productivity of Leucaena leucocephala.
Animal Feed Science and Technology, 60: 29-41.
Göhl B 1998: Tropical feeds. Food and Agriculture Organization. Software developed by Oxford Computer. Speedy A. and Waltham N. Version 8.
Gohl B and Thomke S 1976: Influence of barley tannins on the digestibility of crude protein. Poultry Science, 55: 2369-2374.
Graham H, Inborr J and Åman, P 1991: Definition and Analysis of Dietary fibre: Effect on Nutritional evaluation. Proceedings Vth International Symposium on Digestive Physiology in Pigs. Wageningen (Doorwerth), Netherland. EAAP. Publication 54: 401-404.
Hang D T, Lai N V, Rodriguez L and Ly J 1997: Nitrogen digestion and metabolism in Mong cai pigs fed sugar cane juice and different foliages as source of protein. Livestock Research for Rural Development 9(2). http://cipav.org.co/Irrd/Irrñ/2/Hang92.htm
Jain S K, Gujral G S and Vasudevan P 1987: Potential utilization of water spinach (Ipomoea aquatica). Journal of Scientific Indian Research, 46: 77-78.
Jondreville C, Van Der Broecke J, Grosjean F, Van Cauwenberghe S and Gatel F 2000:Ileal true digestibility of amino acids in wheat milling by-products for pigs. Annales Zootechnie, 49: 55-65.
Jones R J, Blunt C G and Holmes J H G 1976:Enlarged thyroid
glands in cattle grazing Leucaena pastures. Tropical Grasslands, 10:
113-116.
Journey W K, Skillicorn P and Spira W 1991: Duckweed aquaculture. A new aquatic farming system for developing countries. World Bank Technology Division. pp. 35
Kennelly J J and Aherne F X 1980: The effect of fibre in diets formulated to contain different levels of energy and protein on digestibility coefficients in swine. Canadian Journal of Animal Science, 60: 717-726.
Kidder D E and Manner M J 1978: Digestion in the pig. Scientechnica Bristol. 201 pp.
Kumar R and Singh M 1984: Tannins, their adverse role in ruminant nutrition.Journal of Agriculture and Food Chemistry 32: 447-453.
Langer P 1966: Antithyroid action in rats of small doses of some naturally occurring compounds. Endocrinology, 79: 1117-1122.
Larbi A, Dung D D, Olorunju P E, Smith J W, Tanko R J, Muhammad I R and Adekunle I O 1999: Groundnut (Arachis hypogaea) for food and fodder in crop-livestock systems: forage and seed yields, chemical composition and rumen degradation of leaf and stem fractions of 38 cultivars. Animal Feed Science and Technology, 77: 33-47.
Laswai G H, Ocran J N, Lekule F P and Sundstøl F 1997: Effects of dietary inclusion of Leucaena leaf meal with and without ferrous sulphate on the digestibility of dietary components and growth of pigs over the weight range 20 - 60kg. Animal Feed Science and Technolog,y 65: 45-57.
Lee K C and Hutagalung R I 1972:Nutritional value of
tapioca leaf for swine. Malaysian Journal of Agricultural Research, 2:
38-47.
Leng R A, Stambolie J H and Bell R 1995: Duckweed. A potential high-protein feed resource for domestic animals and fish. Proceedings 7th AAAP Animal Science Congress. Improving Animal Production Systems Based on Local Feed Resources pp.103-117.
Lenis N P, Bikker P, van der Meulen J, van Diepen J Th. M, Bakker J G M and Jongbloed A W 1996: Effect of dietary Neutral Detergent Fiber on ileal digestibility and portal flux of nitrogen and amino acids and on nitrogen utilization in growing pigs. Journal of Animal Science, 74: 2687 - 2699.
Lindberg J E, Cortova Z and Thomke S 1995: The nutritive value of lucerne leaf meal for pigs based on digestibility and nitrogen utilization. Acta Agriculturae Scandinavica Section A, Animal Science, 45: 245-251.
Loc N T 1996: On-farm and on-station evaluation of cassava root silage for fattening pigs in Central Vietnam. MSc. Thesis, Swedish University of Agricultural Sciences.
Low A G 1982: Digestibility and availability of amino acids from feedstuffs for pigs: A Review. Livestock Production Science, 9: 511-520.
National Academy of Sciences 1977: Leucaena: promising forage and tree crop for the tropics. National Academy of Sciences, Washington, DC, 115 pp.
NRC 1998: Nutrient requirements of swine. Tenth revised edition. Washington D.C
Oke O L 1978: Problems in the use of cassava as animal feed. Animal Feed Science and Technology, 3: 345-380.
Oke O L 1994: Eliminating cyanogens from cassava through processing: Technology and tradition. In: Acta Horticulturea Cassava Safety 375. Proceedings of an International Workshop in Nigeria. 163-174
Phuc B H N, Ogle B and Lindberg J E 2001:
Nutritive value of cassava leaves for monogastric animals. International
Workshop "Current Research and Development on use of Cassava as Animal Feed".
31-40.
http://www.forum.org.kh/~mekarn/proc-cass/phuc.htm
Phuc B H N and Lindberg J E 2000: Ileal and total tract digestibility in growing pigs fed cassava root meal diets with inclusion of cassava leaves, leucaena leaves and groundnut foliage. Animal Science, 71:301-308.
Phuc B H N, Ogle B and Lindberg J E 2000:Effect of replacing soybean
meal with cassava leaf protein in cassava root meal based diets for growing pigs
on digestibility and N retention.
Animal Feed Science and Technology, 83: 223-235.
Phuc B H N, Ogle B and Lindberg J E 2001:Nutritive value of cassava leaves for monogastric animals. International workshop on Current Research and Development on the use of Cassava as Animal Feed. 31-40.
Phuc B H N 2000:Tropical Forages for Growing Pigs. Digestion and
Nutritive Value. Doctor's dissertation. ISSN 1401-6249, ISBN 91-576-5755-6.
Swedish University of Agricultural Sciences, Uppsala, Sweden.
Ravindran V 1990: Cassava leaf meal. In: Non-Traditional Feed Sources for Use in Swine Production. (Eds), Thacker PA and Kirkwood R N Butterworths, Boston. pp 91-101.
Ravindran V 1993: Cassava leaves as animal feed: Potential and limitations. Journal of the Science of Food and Agriculture, 61: 141-150.
Rogers D J and Milner M 1963: Amino acid profile of manioc leaf protein in relation to nutritive value. Economic Botany 17: 211-216.
Sarwat S V, Kakala S N and Kategile J A 1998: Performance of growing-finishing pigs when fed diets containing fresh cassava leaves and roots. East African Agriculture and Forestry Journal, 53(3): 111-115.
Shihombing D T H, Crownwell G L and Hays V W 1971: Effect of added thiocyanate and iodine to corn-soyabean meal diets on performance and thyroid status of pigs. Journal of Animal Science, 33: 1154-1159.
Sundaresan S, Amma C S E and Nambisan B 1987:Bitterness in
cassava in relation to cyanoglucoside content. Indian Journal of Agricultural
Science, 57(1): 37-40.
Telek L and Martin F W 1983: Tropical plants for leaf protein concentrate. In: Leaf protein concentrate. (Eds) Telek L and Graham H D. AVI Publishing USA. 81-116
Van Soest P J, Coklin N L and Horvath P J 1987: Tannins in foods and feeds. In: Proceedings of Cornell Nutrition Conference for Feed Manufacturers, Cornell University, Ithaca, New York, USA. pp.115-122.
Van Soest P J 1994: Nutritional ecology of the ruminant. 2nd edition. Cornell University Press, Ithaca, USA.
Van Soest P J and Mason V C 1991: The influence of the Maillard reaction upon the nutritive value of fibrous feeds. Animal Feed Science and Technology, 32: 45-53.
Wayman O, Iwanaga I I and Hugh W I 1970: Fetal resorption in swine caused by Leucaena Leucocephala (Lam) de Wit. in the diet. Journal of Animal Science 30: 583-588.
Wood T 1965: The cyanogenic glucoside content of cassava and cassava products. Journal of the Science of Food and Agriculture, 16: 300-305.