Ensiling
leaves of Taro (Colocasia esculenta) with sugar
cane molasses
Malavanh Chittavong, T R Preston* and R B Ogle**
National University of Laos
malavanc@yahoo.com
*Finca Ecológica, TOSOLY, Socorro Colombia
trpreston@mekarn.org
**Swedish University of Agricultural Sciences, Uppsala
Brian.ogle@huv.slu.se
Abstract
An experiment was carried out at laboratory scale to determine the optimum level of molasses for ensiling taro (Colocasia esculenta) leaves. The leaves were collected from Nongveng village, near Vientiane City in Laos, and were chopped into small pieces (2 to 3 cm) and ensiled in plastic bags (capacity 2 kg) with levels of sugarcane molasses of 0, 2, 4 and 6% (dry matter [DM] basis). The mean total sugar content (°Brix) of the molasses was 77. Each molasses level was repeated five times, corresponding to ensiling periods of 0, 7, 14, 21 and 28 days. At each ensiling date samples were taken for determination of pH, DM, ammonia nitrogen (NH3-N), water extractable DM and N, and total N. Physical characteristics, such as smell and colour, were observed and recorded.
After 7 days the colour for all treatments had changed from green to yellow-brown and was darker at higher levels of molasses. Each treatment had an acceptable smell. The pH values for all treatments were around 6 at day 0 and then quickly fell below 5, the value being dependent on ensiling time and the level of molasses (P<0.05). At day 0 the concentration of NH3-N was very low on all treatments, but from 7 days onwards the concentration had increased with the time of ensiling on all treatments ; the highest value was 5,900 mg/kg DM on the 0% level of molasses at 28 days. The ammonia-N concentration decreased as the level of molasses increased.
A level of 4% molasses and an ensiling
period of between 14 and 21 days appeared to be the most appropriate procedures
for ensiling Taro leaves as determined
by pH, ammonia concentration and water extractable DM and
N.
Key words: ammonia, Colocasia esculenta,
ensiling, leaves, nitrogen, pH, taro, water extractable DM and
N
Introduction
Leaves from Taro (Colocasia esculenta Schott) are
rich in vitamins and minerals. They are a good source of thiamin,
riboflavin, iron, phosphorus and zinc, and a very good source of
vitamin B6, vitamin C, niacin, potassium, copper and manganese.
Oxalic acid may be present in the corm, however, and especially in
the leaves (http://en.wikipedia.org/wiki/Taro).
The leaves can be chopped and ensiled to considerably reduce
undesirable substances in Taro, which thus becomes more appetising.
Ensiling is the preservation of forage (or crop residue or by-product) of high moisture content based on a lactic acid (ideally) fermentation under anaerobic conditions (Moran 2005; McDonald et al 2002). It has been shown that ensiled fish and shrimp by- products can be preserved for several months (Levin 1994; Ly et al 2000). The silage can then be used as feed for livestock (Lien et al 1994; Borin et al 2000; Ly et al 2000; Le Duc Ngoan 2002). Recent research has shown that Golden Apple Snail flesh can be successfully preserved for at least 24 weeks by ensiling with an additive mixture of molasses and rice bran and then successfully used as feed for growing pigs (Lampheuy and Ogle 2005). The main function of a silage additive is to increase the nutritional value or improve fermentation (Ohio Sate University Extension 2001). Molasses is a good and cheap additive and is available in Vientiane Province. It has a high soluble carbohydrate content of about 700 g/kg DM and has been shown to increase the dry matter and lactic acid content, and to reduce the pH and ammonia levels in treated silages (McDonald et al, 2002). However, according to Ohio State University Extension (2001), molasses should not be added to wet silage (<35% dry matter) because of increased seepage losses .
The objective of this experiment was
to determine the optimum level of molasses additive for ensiling
Taro leaves.
Materials and Methods
Location
The experiment was conducted in the Laboratory of the Faculty
of Agriculture, National University of Laos (NUOL), Vientiane City,
Laos, from May to June 2006.
Procedure
Leaves of Taro (Colocasia esculenta) were
purchased from Nongveng village, near Vientiane City (Photo 1). They were chopped into small pieces (2 to 3 cm) and ensiled in
plastic bags (capacity 2 kg) with levels of sugar cane molasses of
0, 2, 4 and 6% (DM basis). The bags were sealed to prevent air contamination and then put into plastic buckets to exclude mice and
prevent external mechanical damage and stored at room temperature
(20-30oC). The total sugar content (°Brix) of the
molasses was 77. Each molasses level was repeated five times,
corresponding to ensiling periods of 0, 7, 14, 21 and 28
days.
|
|
|
Photo 1: Taro (Colocasia esculenta) in Nonveng village
Measurements
Samples were taken to analyze for total and water extractable DM and N, and fermentation characteristics such as pH and NH3-N. Water soluble DM and N were determined by the methods described by Ly and Preston (1997). Physical characteristics, such as smell and colour, were observed and recorded. N was determined according to AOAC (1990) and DM by micro-wave radiation (Undersander et al 1993).
Statistical analysis
The data were analyzed using the general linear model option of Minitab (version 14) ANOVA software (MINITAB 2000). Sources of variation were treatments and error.
Results
Chemical composition of the Taro leaves and
molasses
The chemical characteristics of the fresh Taro leaves and molasses are shown in Table 1.
|
Table 1. Chemical characteristics of the fresh taro leaves and molasses |
||
|
|
Fresh taro leaves |
Molasses |
|
Dry matter |
16.0 |
66.1 |
|
% of DM |
||
|
Crude protein |
22.5 |
0.60 |
|
Crude fiber |
18.3 |
- |
|
Total sugars (brix º) |
|
77.0 |
|
Ash |
11.7 |
33.4 |
Physical characteristics
Initially, the Taro leaf silage at 0% molasses had a green color , but for the other treatments the colour rapidly started
to change from green to brown. After 7 days the color for all
treatments had changed to yellow-brown, and was darker at higher
levels of molasses. The color did not change further in the
treatments with molasses added, but the colour of the Taro leaf
silage at the 0% level changed to yellow-brown at 21 days, and
became even darker at 28 days. All silages had a good smell
throughout.
Changes in chemical composition and pH with time of
ensiling
The pH values for all treatments were around 6.0 at day 0 and then fell below 5.0 in all treatments after 14 days, except for the 0% molasses treatment (Figure 1; Table 2). The pH values further decreased with time of ensiling (P<0.05) and level of molasses (P<0.05). For example, at the 4% level of molasses the pH value was 4.0 at 21 days (Figure 1). The DM content of al the silages was some 6 percentage units higher than in the fresh leaves.
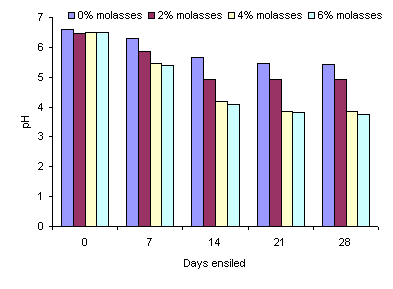
Figure 1. Effect of ensiling period and level of molasses
on the pH of ensiled taro leaves
At day 0 the concentration of NH3-N was very low on all treatments (Figure 2). After 7 days the concentration increased with the time of ensiling on all treatments (P<0.05); the highest value recorded was 1.3 g/kg DM on the 0% level of molasses at 28 days. The ammonia-N concentration decreased in proportion to the level of molasses (Figure 2; Table 2).
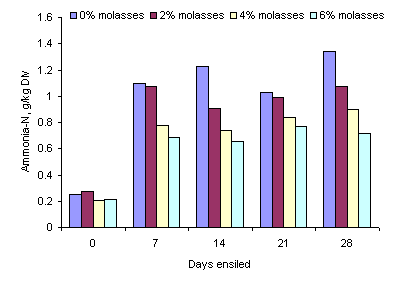
Figure 2. Effect of time of ensiling and level of molasses
on ammonia nitrogen in ensiled taro leaves
The proportion of the DM solubilised by water extraction was not affected by ensiling time but increased with the increasing level of molasses (P<0.05) (Table 2 and 3 and Figure 3).
|
Table 2. Effect of ensiling time on the chemical composition of ensiled Taro leaves |
|||||||
|
Parameter |
Days |
SEM |
Prob. |
||||
|
0 |
7 |
14 |
21 |
28 |
|||
|
pH |
6.5 |
5.8 |
4.7 |
4.5 |
4.5 |
0.17 |
0.000 |
|
DM, % |
22.0 |
24.0 |
20.6 |
20.8 |
20.9 |
0.50 |
0.002 |
|
NH3-N, mg/kg DM |
236 |
908 |
879 |
906 |
1006 |
54.1 |
0.000 |
|
N, % |
2.5 |
2.7 |
2.8 |
2.8 |
2.9 |
0.05 |
0.001 |
|
WV-DM, % |
34.6 |
36.1 |
35.3 |
43.6 |
38.8 |
2.25 |
0.086 |
|
WV-N, % |
27.9 |
40.5 |
38.5 |
40.0 |
41.0 |
2.49 |
0.014 |
|
Table 3. Effect of molasses level on the chemical composition of ensiled Taro leaves |
||||||
|
Parameter |
Molasses, % |
SEM |
Prob. |
|||
|
0 |
2 |
4 |
6 |
|||
|
pH |
5.9 |
5.4 |
4.8 |
4.7 |
0.15 |
0.000 |
|
DM, % |
22.2 |
20.6 |
21.4 |
22.5 |
0.44 |
0.037 |
|
NH3-N, mg/kg DM |
989 |
863 |
689 |
607 |
48.4 |
0.001 |
|
N, % |
2.8 |
2.8 |
2.7 |
2.7 |
0.05 |
0.061 |
|
WV-DM, % |
29.3 |
30.8 |
44.0 |
46.6 |
2.01 |
0.000 |
|
WV-N, % |
30.8 |
35.2 |
42.2 |
42.2 |
2.23 |
0.008 |
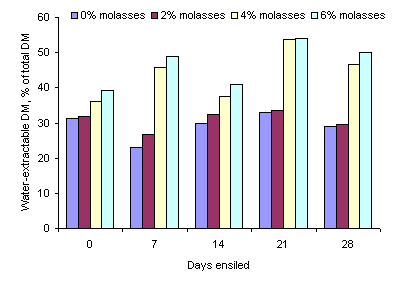
Figure 3. Effect of length of ensiling period and level of molasses on water-extractable DM in ensiled taro leaves
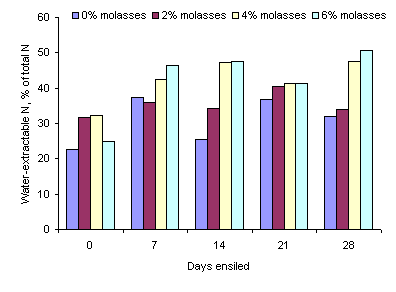
Figure 4. Effect of length of ensiling
period and level of molasses on water-extractable N in ensiled taro leaves
The proportion of the total N
extracted by water increased both with length of ensiling period and the level of
molasses (Tables 2 and 3 and Figure 4)., and was highest at the 4 and 6% levels
(P<0.05).
Discussionduring transport
According to McDonald et al (2002), if the material to be ensiled has a relatively low DM content, it is advisable to use an additive with high dry matter content, which was the case in the present study. The higher DM content of the silage (20 to 24%) compared with the fresh leaves (16% DM) was probably the result wilting during transport from the village and subsequent chopping at the University. The pH values for all treatments were around 6.0 initially, and then fell quite rapidly, because any oxygen trapped between the forage particles is eliminated as a result of the respiration of the plant material and the anaerobic activities of bacteria start to dominate. The plant enzymes are also active during this initial phase, provided the pH is still within the normal range for fresh material (pH 6.0-6.5) (Moran 2005). However, after 14 days the pH values had fallen to below 5.0, except in the treatment without any addition of molasses. A successful fermentation will see the number of lactic acid producing bacteria dominate under anaerobic conditions and when there is a readily-fermentable substrate such as molasses, reducing the pH to 3.5 to 4.5 (Moran 2005), as was also the case in the present study.
During the 28 days of ensiling, there were only very slight
changes in the chemical composition of the Taro leaf silage, which
is in agreement with several previous studies. McDonald et
al (2002) reported that losses of DM of less than 5% during
ensiling are acceptable. The slight decrease in CP content was
because there is normally some de-amination of amino acids that
occurs during fermentation (McDonald et al 2002). Lin et al (1988) also concluded that the general nutrient values
(including metabolizable energy), fatty acid composition and amino
acid contents (including the proportion of essential amino acids)
in silages of mixtures of sweet potato roots and maize meal did not
change during ensiling. Ruiz (1982) showed that the dry matter
content of sweet potato foliage silages did not change by adding
roots (up to 1.2%) or urea (up to 1.6%).
The increase in the concentration of ammonia-N during the first 7 days of ensiling are in accordance with the report of McDonald et al (2002) that proteolytic organisms (mainly Clostridia) are active while the pH is still relatively high (falling only from 6.5 to 5.8 in the first 7 days) resulting in breakdown of protein to amino acids, amines and ammonia. After 7 days there was no further increase in ammonia-N concentration. The reduction in ammonia-N due to addition of molasses was probably because the higher the concentration of molasses the faster was the reduction in the pH of the silage (see Figure 1). Part of the increase in water soluble DM due to addition of molasses can be explained by the effect of the molasses per se as most of the compounds in molasses (sugars and minerals) are water-soluble. However, at the 4 and 6% molasses levels the increase in water-extractable DM was almost 100% which implies that under these conditions (4 and 6% molasses) the ensiling process had stimulated partial breakdown of some of the components in the taro leaves. Similar effects were seen in the proportions of water-extractable N which increased by more than 50% due to the addition of the molasses. It will be interesting to determine if these changes in water-extractable DM and N are reflected in increased in vivo digestibility.
Conclusions
-
A level of 4% molasses and an ensiling period of from 14 to 21 days appeared to be the most appropriate for ensiling taro leaves, as determined by pH and ammonia concentration..
Acknowledgements
The authors wish to acknowledge the MEKARN project, financed by
Sida/SAREC, for supporting this experiment. Special thanks are
expressed to Mr Bounlerth for helping in the laboratory. We also
wish to thank the Department of Livestock and Fisheries, National University of
Laos, for providing part of the facilities to carry
out the experiment.
References
AOAC 1990: Official Methods of Analysis. Association of Official Analytical Chemists. 15th Edition. Arlington pp 1230.
Borin K, Sim Chou and Preston T R 2000: Fresh water fish silage as protein source for growing-fattening pigs fed sugar palm juice. Livestock Research for Rural Development 12 (1): http://www/cipav.org.co/lrrd/lrrd12/1/borin121.htm
Lin Y H, Huang T C and Huang C 1988: Quality improvement of sweet potato (Ipomoea batatas L. Lam.) roots as feed by ensilage. British Journal of Nutrition, 60: 173-184.
Levin R E 1994: Lactic acid and propionic acid fermentation of fish hydrolyzates processing: Biotechnological Applications, ed. A.M. Martin. London.
Lien L V, Sansoucy R and Thien N 1994: Preserving shrimp heads and animal blood with molasses and feeding them as a supplement for pigs. Proceeding of SAREC Workshop, Ho Chi Minh City.
Ly J and Preston T R 1997: An approach to the estimation
of washing losses in leaves of tropical trees. Livestock Research
for Rural Development (9) 3: http://www.cipav.org.co/lrrd/lrrd9/3/ly931.htm
Le Duc Ngoan 2002: Evaluation of shrimp by-products for pigs in central Vietnam. Doctoral thesis, SLU, Uppsala.
Lamphuey K and Ogle B 2005: Evaluation of the Nutritive Value of Ensiled and Fresh Golden Apple Snails (GAS) (Pomacea spp) for Growing Pigs. Master of Science thesis, Department of Animal Nutrition and Management, Swedish University of Agricultural Sciences. http://www.mekarn.org/msc2003-05/theses05/lamp1.pdf
McDonald P, Edwards R A, Greenhalgh J F D and Morgan C A
2002:Animal Nutrition. Sixth Edition. Longman Scientific and
Technical, Harlow, Essex, England.
Moran J 2005: Feeding management for smallholder dairy farmers in the humid tropics. Department of Primary Industries, Landlinks Press, pp 312.
Nguyen Hoa Ly, Le Van An and Le Duc Ngoan 2000:
Evaluation of ensiled shrimp by-products for fattening
pigs.Workshop-seminar "Making better use of local feed resources"
SAREC-UAF. http://www.mekarn.org/sarpro/ngoan.htm
Ohio State University Extension 2001: Silage
Additives, Department of Horticulture and Crop Science, Columbus,
Ohio, USA. http://ohioline.osu.edu/agf-fact/0018.html
MINITAB 2000: Minitab Reference Manual, release 14.
Ruiz M E 1982: Sweet potatoes (Ipomoea batatas (L) Lam) for beef production: Agronomic and conservation aspects and animal responses. In: Sweet Potato. Asian Vegetable Research and Development Center. pp. 439-452.
Undersander D, Mertens D R and Theix N 1993: Forage analysis procedures. National Forage Testing Association. Omaha pp 154.

