Digestion in the rabbit -a new look at the effects of their feeding and digestive strategies
R A Leng
PO Box 361, Coolum Beach 4573
Queensland, Australia
rleng@ozemail.com.au
Abstract
Rabbits are hind gut fermentors that,
because of their high surface to body weight ratio, have a relatively high
maintenance energy requirement. Rabbits given concentrate based diets (energy
dense) need to consume 5% or more but with forage alone this is increased to
over 8% of body weight on a dry matter basis.
Rabbits efficiently utilise fibrous
feed by courtesy of their feeding and digestive strategies. They are highly
selective when given forage, free choice and in quantity. Their digestive
strategies include initial enzymic digestion in acidic followed by alkaline
medium of the stomach and small intestines respectively, followed by
fermentation of feed residues in the caecum large intestines.
The rabbit has the ability, through
specialised musculature of the large intestine, to direct slowly fermented
fibrous feed into the colon for excretion in the hard faeces. The same mechanism
separates small particles (more digestible fiber) and soluble components of
digesta leaving the ileum into the caecum where it remains in a buffered medium
supporting bacterial growth. The rabbit quickly "sieves" indigestible or slowly
fermentable fibers which would slow its feed intake were it to enter the caecum.
Hard faeces are produced some 4 hours after a meal.
The caecum is relatively large and
the products of fermentation are similar to the products in foregut fermentors
such as the ruminant- that is volatile fatty acids (VFA) and microbial cells. A
number of reasons are put forward for the microbial growth being highly
efficient in the caecum of rabbits on forage-based diets yielding a higher ratio
of cells to VFA than may occur in fermentation in the forestomach of ruminants.
Considerable VFA are apparently absorbed by the rabbit but the availability of
the essential amino acids of microbial protein depends on the subsequent
consumption of soft faeces or caecotropes.
Caecotropes are formed from the
contents of the caecum as they pass through the large intestine and colon of the
rabbit. The feed residues and culture medium are voided from the caecum about
8hours after a meal. The digesta with its microbes are formed into pellets by
dehydration and coated with a mucous membrane as they pass along the colon.
These soft pellets or caecotropes are retrieved as they pass from the anus and
are swallowed by the animal without disrupting their membranes; they are also
buffered towards a neutral pH . These pellets enter the acidic stomach and
reside in the fundus portion for several hours. The membrane and the buffered
contents maintain the pellet contents at close to neutrality. They finally
disintegrate with increasing feed intake and the contents are then subject to
gastric and intestinal digestion.
The concept is floated here to
explain why the process of caectrophy may be a highly efficient strategy to
utilise microbial cells produced in the hind gut. Recent studies have indicated
the involvement of a group of enzymes termed lysozymes in the degradation of
bacterial cells. Many secretions of the body contain lysozymes, but it appears
that foregut fermentors such as ruminants have evolved lysozymes produced in the
abomasum as part of their digestive strategies. There is also evidence that
rabbits have also evolved a similar mechanism, but in this case the lysozyme is
secreted by the colon wall as the membrane is secreted onto the soft pellet.
Lysozymesare a group of enzymes that specifically digest the mucopolysaccharide, peptidoglycan of bacterial cell walls. The peptidoglycan envelop appear to slow the potential digestion of bacterial cells and the short small intestine of the rabbit may limit efficiency of digestion of caecal microbes. It is hypothesised here that lysozyme is involved in the digestion of bacteria in the caecotropes in the stomach.
To enhance the digestion of bacteria
it is also hypothesised that bacteriophage action is also involved in lysis of
bacteria in both the stomach of the rabbit and the abomasum of the ruminant.
The efficient use of forage based
diets, and capacity to breed with multiple offspring, demarcates a special role
for the forage-fed rabbit when oil prices rationalise the use of grain for
intensive animal production
Key words: bacteria, bacteriophage, caecotrophy, feeding strategy, fermentation, forages, lysozymes,peptidoglycan, rabbits, Yatp
Introduction
Rabbits are herbivores and are classified as hindgut
(caecum and colon) fermentors. In nature they are highly selective feeders and
they can efficiently digest a wide range of simple and complex carbohydrates by
curtsey of their digestive strategy. The rabbit has an efficient monogastric
mode of digestion that is followed by fermentation of 'selected' cellulose feed
and endogenous materials in the caecum through the action of a resident
bacterial ecosystem comprised primarily of
Bacteroidesspp
Because of a small body size [large surface area to
weight] the rabbit has a high metabolic rate and therefore a relatively high
maintenance energy requirement particularly when compared to ruminants and
equines. Forage intake in the former is constrained by rate of comminuting feed
to small particles in the rumen: The horse on the other hand compensates for low
digestibility of forage by eating more feed and passing this through the gut
rapidly; sacrificing digestibility to meet energy demand. The pig is limited in
its use of forage by the slow movement of cellulosic materials through the large
intestine which feed back to limit intake.
On concentrate diets, feed intake in the rabbit has
to be in excess of 5% of live weight per day (Irlbeckel 2001) and as high as
8-10% of live weight per day on forage based diets (Pok Samkol et al 2006b) to
ensure nutrient availabilities are above maintenance. These levels of intake are
achieved through a special adaptation of digestive processes that allows the
rabbit to efficiently digest non-fibrous carbohydrates, but quickly exclude from
its digestive tract the relatively indigestible fiber in a diet and at the same
time preserve digestion of readily fermented fiber [polysaccharides] in the
caecum.
The rabbit caecum is very large, compared with the
rest of the gut (Stevens and Hume 1995) and forms a spiral that fills the
abdominal cavity (see Figure 1).
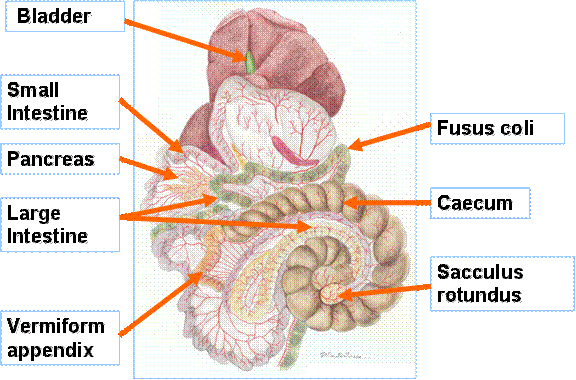
Figure 1:The digestive
tract of the rabbit
The caecum has a capacity 10 times that of the
stomach, and occupies about 40% of the gastrointestinal tract in concentrate fed
rabbit but this may be greater in forage fed animal (Jenkins 1999. Rabbits
utilize a mechanism to expel slowly fermented fiber from the gastrointestinal
tract, whilst retaining readily fermented materials and endogenous nitrogenous
components of the digesta leaving the ileum. Consumption of soft faeces [caecotropes]
produced from the materials that pass through the caecal cycle also compensates
for low or poor-quality protein in a diet by recycling, via fermentation in the
caecum, endogenous protein secretions and sloughed epithelial cells from the
small intestine (Carabano and Piquer 1998).
The specialized digestive strategies of rabbits
Feed undergoes enzymatic digestion in both the
stomach and small intestine as the digesta moves through the tract. The small
intestine in the rabbit is relatively short so only readily digestible materials
are extracted such as sugars, soluble protein and particulate starch. Adult
rabbits appear to have little capacity to digest fat in the small intestine and
lipase activity is restricted to the caecum (Marounek et al 1995). High amounts
of fat in a diet may therefore limit cellulose fermentation in the caecum as
shown for the forestomach of ruminants (see Devendra and Lewis 1974). Fat
deposition on fiber,increasing its density, may also be a factor in allowing the
separation of the course fibers away from the caecum, and their subsequent
excretion as components of the hard faeces.
When digesta from the ileum enters the large
intestine, muscular contractions facilitate the separation of slowly digestible
fiber from other materials including non fiber fractions (eg: protein and
soluble carbohydrates) and small fibrous components. A series of strong
peristaltic contractions move fibrous particles through the colon towards the
anus and anti peristaltic waves move liquid and small particles back into the
caecum. In this way the rabbit separates and concentrates digesta fractions
which are readily degradable for preferential fermentation in the caecum ( Lebas
et al 1997; Carabano and Piquer 1998); particle size and fluid density aid
separation (Cheeke 1994). The relatively indigestible fiber components that
separate out are voided quickly as hard faeces (Figure 2) about 4 h after
consumption of a meal (Cheeke 1994).

Figure 2: Caecotropes (left of
picture) from the fundus compartment of the abomasum and hard faeces (right of
picture)
Fermentation of the materials that are directed into
the caecum, proceeds for some further 4 hours. During this time, contractions of
the caecum move the lumen digesta contents towards the blind sac and back and at
the same time mixes the contents. Fermentation of solubles and fibrous materials
is likely to be efficient in terms of microbial growth since the residence time
of digesta is only 4h compared with retention times of feed in the rumen often
greater then 12h.
Small discrete digesta samples are periodically
forced by muscular contractions into the large intestines where some moisture is
absorbed along with some [most?] VFA (Stevens and Hume 1995). As the digesta
from the caecum is propelled towards the anus it forms into pellets which are
coated with a mucous membrane secreted by the cells of the colon wall to become
caecotropes. The caecotrope is voided from the body approximately 8 hours after
consumption of a meal (Cheeke 1994). The rabbit recognizes the caecotrope to be
voided and consumes it directly from the anus. This practice of consuming
caecotropes is called coprophagia, or caecotrophy. Under natural or grazing
conditions, caecotrophy usuallyoccurs during the day and feeding is restricted
to the night time. Hard faeces and soft faeces are excreted in a circadian
rhythmic pattern(Carabano and Piquer 1998; Lebas et al 1997). If a rabbit is
equipped with a collar preventing caecotrophy, the digestion of the diet is
significantly reduced and growth rates are often decreased by some 50% (Figures
3a and b).
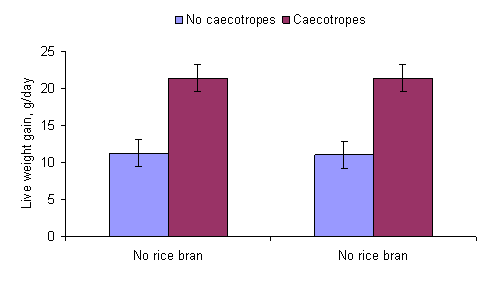
Figure 3a: Effect of access or not
to caecotropes on the growth rate of rabbits fed water spinach with or without
supplementation with rice bran (ChievPhiny and Lampheuy 2006).
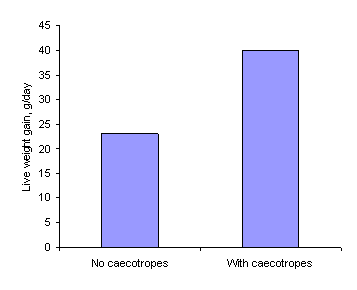
Figure 3b.Food intake and weight
gain in New Zealand White rabbits fed a concentrate pellet (Stephens 1977)
When allowed to select their diet, rabbits will
select the least lignified, succulent plant parts or the plant parts that are
most nutrients dense and highest in sugar. This feeding strategy allows the
animal to meet the dietary requirements for their high metabolic rate (Cheeke
1994). Rabbits eliminate relatively indigestible fiber as quickly as possible
from their gastrointestinal tract.In many situations this would be the most
lignified fibrous materials.
Rabbits have high feed intake and fast feed transit
time, which enable them to consume forages and meet nutritional requirements
(see Pok Samkol et al 2006b; Carabano and Piquer1998). Understanding the
digestive physiology of the rabbit and how dietary components affect microbial
growth efficiency in the caecum is a key to proper feeding management.
Caecum microbes and utilization of fiber by the rabbit
When compared with other herbivores, digestion of
structural components of plants such as alfalfa in mixed concentrate based diets
for rabbits is relatively low (14% for alfalfa hay in rabbits compared with 44%
in cattle, 41% in horses, and 22% in pigs) (McNitt et al 1996). The actual crude
fiber component of most forages fed to rabbits in industrialized countries is
only 20 to 25% (McNitt et al 1996), depending on forage source and its maturity.
Other non fiber fractions of forage such as protein, and soluble carbohydrates
are easily digested by rabbits. In concentrate-fed rabbits supplemented with
forages such as lucerne the dietary fiber is structural and often closely
associated with lignin and has a critical role in maintaining gut health,
stimulating gut motility (insoluble fiber only), reducing fur chewing, and
preventing enteritis (McNitt et al 1996; Brooks 1997). Concentrate based diets
with less than 20 to 25% fiber result in reduced gut motility, reduced
caecotrope formation, prolonged retention time in the hindgut, and often
enteritis and diarrhea (Cheeke 1994; Jenkins 1999).
In the forage-fed rabbit the diet is a mixture of
soluble sugars, protein and fiber that may be relatively un-lignified or highly
lignified according to source. For instance the rabbit fed water spinach or tree
leaves consumes little lignified fiber. In these diets it is the soluble
components and readily fermented structural carbohydrates that provide the
energy and protein requirements .On the other hand when fed grasses or tall
growing legumes the fiber can be highly lignified as it supports the upright
growth of the plant.
Composition of the hard feces and the caecotrope is
influenced by the diet. If dietary fiber concentration increases, the fiber
composition of the faecal pellets also increases. Fiber fermentation in
concentrate-fed rabbits does not seem to be enhanced by coprophagia (as cited by
Cheeke 1994) but it is clearly influenced when rabbits receive only forage as
the basis of the diets (ChievPhiny and Lampheuy 2006).
Stoichiometry of fermentation in the rabbit's caecum and the forestomach of ruminants
Fermentation in the caecum results in an increase in
microbial biomass and the production of volatile fatty acids [VFA] in a similar
way as occurs in the foregut fermentation of ruminants. However there are
potentially significant differences in the efficiency of net microbial growth in
the rabbit caecum as compared with the ruminant forestomach in both the forage
and concentrate fed animal
In rabbits fed a traditional alfalfa/maize diet,
acetate is the primary volatile fatty acid (VFA) produced by microbes, with more
butyrate than propionate being formed. Microbes in the rabbit's caecum produce
more VFA on starch-based diets than on forage diets (Cheeke 1994), which may be
linked with the efficiency of microbial growth rather then differences in total
dry matter digestion by fermentation. Stevens and Hume (1995) indicate that VFA
provide a major energy source in the rabbit colon.
Utilization of Protein
The traditional view point
Even though amino acids from bacterial protein may
be available via coprophagia (especially lysine, sulfur amino acids, and
threonine; Carabano and Piquer 1998), research has shown that microbial protein
plays only a minor role in meeting a rabbit's protein and amino acid needs on
diets based on concentrates (McNitt et al 1996). The majority of microbial
protein utilized by the animal is digested in the colon (Stevens and Hume 1995),
presumably once the caecotrope disintegrates and its contents are released in
the stomach. Caecotropes, however, contain approximately 28% crude protein
(Stevens and Hume 1995) which may indicate they are approximately 50% bacterial
cells as bacterial cells from the rumen are 50-60% crude protein. The low
apparent utilization of microbial protein may be caused by the diet. Recent
studies with rabbits on forage-based diets indicate that prevention of
caecotrope consumption has big effects on growth of rabbits, which is not in
line with the concept that they supply little extra nutrients as VFA and
essential amino acids of microbial origin. .
Rabbits candigest 75 to 85% of lucerne protein,
whereas pigs digest less than 50% (McNitt et al 1996).Urea is recycled by the
rabbit large intestine in a manner similar to that occurring in the rumen
(Stevens and Hume 1995) and urease levels are highest in the caecum (Marounek et
al 1995). Urea is converted to ammonia in the gut, and if the content of
carbohydrate is low, the ammonia may be absorbed and this could result in
toxicity. When an animal is fed a low-energy diet, caecotrope ingestion is
maximized (Jenkins 1999). Low levels of dietary protein fed to rabbits increase
caecotrope consumption and high levels of protein decrease consumption, which
seems to be a protein sparing mechanism (Cheeke 1994). Coprophagia has been
found to increase protein digestibility (50 vs 75 to 80% for alfalfa) of forages
in rabbits
An alternative view point of forage utilization in the forage fed rabbit
In any fermentative system VFA production and
microbial growth are linked; the energy for microbial growth is provided by ATP
produced when carbohydrate and protein are degraded to VFA. The microbial
polymers or building blocks for microbial cells are synthesized from the
intermediates of glycolysis and VFA formation (Preston and Leng 1987). Y-atp
(Bauchop and Elsden 1960)is a useful description of the efficiency of microbial
growth. It is defined as the g dry cells produced in fermentation per mole of
ATP available in the conversion of organic matter to VFA
Brief outline of the relationship between Yatp and the cells available for digestion
In the rumen Yatp is much reduced by the long
retention time of feed particles together with a high maintenance requirement of
the microbes. In addition a considerable amount of lysis of microbes occurs from
the action of lytic phage, autolysis and predation by the protozoa (Wells and
Russell 1996; Klieve and Swain 1993; Leng and Nolan 1984). Predation of protozoa
can be significant (Bird and Leng 1984) often decreasing the net availability of
microbial protein by 25-35% (Bird and Leng 1984) but, in addition, protozoa have
a high maintenance energy requirement (use ATP for maintenance rather then
growth) and are retained in the rumen for longer periods than indicated by rumen
turnover time (Weller and Pilgrim 1974). Most measurements for Yatp in the rumen
suggest a value of 8-14 g cells/mole of ATP generated in fermentation, whereas
the theoretical Yatp should be about 26 g cells per mole of ATP. The Yatp for
fermentation in the caecum of rabbits, to my knowledge has not been measured,
but there are a number of indications that many of the inefficiencies in the
rumen do not apply to the rabbit's caecal environment . These include i) the
absence of protozoa; ii) the absence of slowly fermented fiber which has been
rejected through the sieving mechanism in the large intestine; iii) the short
turnover time of the caecum's contents; iv) the removal of anti bacterial
components in the upper digestive tract; v) the enrichment of the medium by
secretions from the small intestine including secreted proteins such as enzymes,
mucous secretions and sloughed cells of intestinal origin; and vi) the small
number of bacterial species which will much reduce inter-species feeding.
With these advantages it is feasible that the end
products of fermentation in the rabbit caecum will be richer in cells and
therefore in protein (bacteria are 50-60% protein) than is the case for rumen
fermentation. This potentially rich source of essential amino acids is then made
available to the rabbit through coprophagia. This idea is supported by the very
high protein content of the caecotrope (28% CP in DM see Stevens and
Hume(1995)and 47% in DM in rabbits fed water spinach leaves or stems as the sole
diet (Pok Samkol et al (2006a)).
A high efficiency of microbial growth depends to a
major extent on a rapid turnover of caecal contents in rabbits reducing
bacterial lysis and bacterial maintenance energy requirements. In the pig given
high fiber diets the bacterial protein from the large intestine is unavailable
to the animal and fermentation is a source of energy substrates in the form of
VFA. A slow turnover of the contents of the large intestine is therefore
beneficial if the pig is on a high fiber diet as lysis of cells and fermentation
of bacterial proteins allows more VFA absorption (Marouneket al 2002)As shown by
Dierick et al (1990) the bacterial growth efficiency in the pigs hind gut is
less then half that in the rumen (13.6 g bacterial N excreted in the faeces per
kg of organic matter apparently fermented compared to 30 g N/kg organic matter
fermented in the rumen). The latter is approximately a third of the theoretical
efficiency of anaerobic bacterial growth.
The sieving and rejection of fibrous feed from entry
into the caecum may have further implications for bacterial growth efficiency in
the caecum. As the largest, intact, fibrous components in digesta entering the
large intestine are likely to be the most lignified, the "sieving" action in the
caecum/colon may remove these preferentially and decrease the lignin content of
organic matter in the caecum relative to that in fiber in the diet or in the
hard faeces. The significance of this is that the release of phenolic compounds
from lignin has been shown to suppress bacterial growth in the rumen (see
Bornemanet al 1986) and reduce digestibility of both starch and cellulose. For
example whole oats and oat hulls with high lignin content often have a much
lower digestibility then varieties that have low lignin content (see Black 2001:
Rowe and Crosbie 1998). Thus a physiological mechanism excluding lignin from the
caecum could potentially improve the efficiency of bacterial growth.
The caecotrope which is taken from the anus directly
and swallowed remains in the stomach for some hours. The tough membrane remains
intact for at least six hours after ingestion. When swallowed the caecotropes
pass to the fundus portion of the stomach (Griffiths and Davis 1963). The
membranes around the pellet and a buffering solution in the pellet control pH
and fermentation seems to proceed even though the rest of the stomach is acid
Cheeke (1994) has suggested that the VFA production is not significant but the
permeability of epithelial cells to VFA is high and so the VFA are probably
mostly absorbed from the caecum, large intestine and colon and at high
efficiencies of growth are in much lower proportions in the end products [cells
and VFA]. The purpose of the caecotrope appears to be to allow a prolongation of
fermentation activity while the caecotrope is resident in the stomach. When
feeding commences and these disintegrate they make available bacterial cells and
micronutrient to be used in intestinal digestion.
Is there another reason for the residence of the
caecotrope in the stomach? The movement of digesta materials through the
stomach- small intestine is quick and the small intestine is short suggesting
that digestion of bacterial cells which are enclosed in a highly resistant
membrane envelop of peptidoglycan, may be too slow allow significant significant
digestion.
The possibility is floated here that caecotrophy may
allow an efficient use of bacterial cellular materials. In the process of
formation of the caecotrope and its retention in the stomach, activation of
temperate phage to its lytic phase in bacterial cells causes bacteria to lyse
improving the digestibility of bacterial protein in the stomach and small
intestine. Protein may also be partially digested by enzymes released during
lysis.
In ruminants lysozyme produced in the abomasum assists in the degradation of the ruminants major essential amino acid supply - the microbes produced in the rumen. Lysozymes are a group of enzymes [muramidases] that hydrolyses beta-1,4-links between N-acetyl-muramic acid and N-acetyl-D-glucosamine in the peptidoglycan of bacterial cell walls .The enzyme is found in tears, saliva, white blood cells and macrophages where it may have a defense role against invasive organisms. A group of lysozymes appear to have a digestive role in the true stomach (abomasum) of ruminants and colobine monkeys and also copraphagic animals. These enzymes degrade the mucopolysaccharide cell walls of bacteria passing from the rumen, allowing the cell contents to be digested by other stomach and intestinal enzymes (Dobson et al 1984; Stewart et al 1987; Irwin and Wilson 1989). The stomach form of lysozyme is endowed with special physiochemical properties that allow it to function in an acidic and protease-rich environment.
In the rabbit, lysozyme produced in the
non-sacculated colon is secreted circadially into the colonic lumen in
association with the production of caecotropes that are destained for ingestion
(Camara and Prieur 1984). Because of the mucous coating of the caecotrope, the
lysozymes are concentrated in the caecotrope. Thus from the time the digesta
leaves the caecum and forms a caecotrope and is then excreted and swallowed, to
the time it disintegrates in the stomach there is a considerable opportunity for
lysozyme to degrade the bacterial cell peptidoglycan. However, the lysozymes
appear to have narrow and acidic pH optima (Ito et al 1994), and therefore they
are more active in the low pH of the stomach. The action of lysozyme could be
considerably enhanced if phage-initiated disruption of the bacterial cell
membrane occurred prior to, following or coinciding with the change in pH as the
caecotrope disintegrates in the stomach
Another interesting fact is that bacteriophages also
contain lysozymes which appear to have a pH optima close to neutral. Phages
infect cells by attaching to the cell via a base plate and forcing their tail
tube into the cell membrane puncturing the outer membrane. Contractions of the
sheath progresses enlarging the pore in the membrane until the lysozyme domains
reach the peptidoglycan layer which they digest (see for more detail Kanamaru et
al 2002). The possibility exists that bacteriophage activity is stimulated in
the close to neutral caecotrope prior to the onset of colonic lysozyme activity
when activated by the acid environment of the stomach,
Large numbers of temperate and lytic phage are
associated with rumen organisms (Orpin and Mann 1974: Klieve and Swain 1993). We
have no knowledge of their presence in caecal fluid organisms but it appears
logical that the phages are present in these organisms since the major bacteria
present in caecal contents [Bacteriodes species] are known to be infected
with phage in the rumen (Keller and Traub 1974).
As an aside to the rabbit, and pertinent to the
ruminant, itappears possible that rumen organisms entering the abomasum would be
killed by the acid environment, and for the bacterial cell envelope of
peptidoglycan to remain intact, temperate phage would quickly multiply and
become lytic phage and assist the lysozymes of the acid abomasum to complete the
disruption of the bacterial cells and enhance the process of their digestion. It
thus seems that similar systems to degrade bacteria have evolved in both foregut
and hind gut fermentors via caecotrophy in the latter.
In summary, in the rabbit the lysozyme that is produce by the colon cell wall but conserved in the caecotrope may play a major role in facilitating the digestion of bacteria in the stomach and small intestine. Elucidating the potential role of phages as aides to bacterial cell digestion needs considerable research
Efficient fermentation (high cell yield relative to VFA production in the caecum) and efficient digestion of bacterial cells by combined action of lysozyme, potentially lytic phage and gastric and intestinal enzymes, may account for a very superior mode of both conserving and utilizing essential amino acids by the rabbit.
The interplay between lytic phage and lysozyme may be more complex as recent studies have indicated that bovine abomasum lysozyme may not be active against gram negative bacteria which have lower concentrations and less complex layers of peptidoglycan then gram positive bacteria. In addition some gram positive bacteria appear to be resistant to abomasum lysozyme (Dominguez-Bello et al 2004). Bacteroides species are gram negative and the population of bacteria in the rumen is normally largely gram negative but this can vary considerably. In vitro digestion of bacteria from the rumen with pepsin- pancreatic indicated that gram negative bacteria were 92% digestible whereas gram positive bacteria were 39%.(Wallace 1983). The implications for digestion of bacteria produced in the caecum and consumed in caecotropes is not clear.
Utilization of starch
High-starch diets are often incompletely digested in
the rabbit small intestine due to rapid transit times (McNitt et al 1996).
Incomplete digestion of starch prior to the large intestine results in the
availability of starch for microbial fermentation (Stevens and Hume 1995).
Excess starch in the gut results in an extremely rapid fermentation with
possible spilling of energy leading to low Yatp. For example, Oba and Allen
(2003) showed that, as the rate of starch digestion increased, the Yatp was
reduced from 60g of microbial N/kg starch digested to approximately 30 g
microbial N/ kg starch digested (see Figure 4).
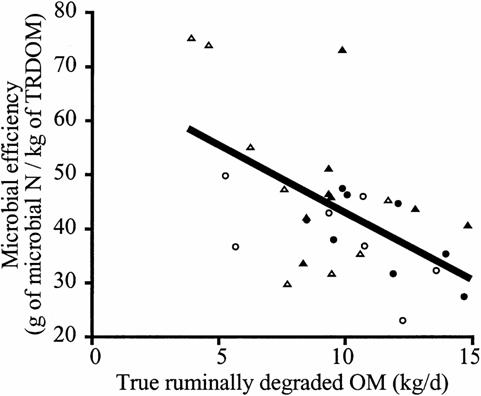
Figure 4.Relationship between rate of starch
digestion in the rumen of cows and microbial efficiency..
Closed circle denotes
high moisture maize in high starch diets; closed triangle denotes dry ground
maize
in high starch diets; open circle denotes high moisture maize in low
starch diets; and open triangle denotes
dry ground maize in low starch diets
(after Oba and Allen 2003) .
The rapid digestion of starch could thus lead to a
much lower microbial cell yield in starch fermentation in the caecum of
concentrate-fed as compared to forage-fed rabbits. If toxin-producing microbes
(primarily Clostridium spiroforme) are in residence, high levels of
starch may lead to enteritis and possible death (McNitt et al1996; Jenkins
1999).
Implications
The use of maize-alfalfa diets for feeding rabbits
is an expensive option, that has developed because of the economic advantages of
industrial production systems in countries with high labour costs, such as the
USA and in the EU. Such feeding systems are designed along similar lines as for
other monogastric animals and may remove the significant advantage of the rabbit
- the ability to utilize caecotrophy as a tool to optimize essential amino acid
availability. The feeding of high-starch diets to rabbits may lead to reduced
microbial growth efficiency in the caecum leading to insignificant improvements
in protein nutrition via caecotrophy. In contrast, on high-forage diets this
process has much more significance if, as suggested in this review, microbial
growth in the caecum is highly efficient.
Feeding a traditional alfalfa and maize diet to
rabbits is not likely to be desirable nor economic in the future as resource
depletion and competition for grain for feed, food and feedstock, forces up the
price of grain (Preston 2006).
The potential feed sources for rabbits are widely
available in most tropical countries in Asia. However, any feeding system should
recognize that the time of supplying feed should not disturb the potential
circadian rhythm of caecotrope production. Considerable research is needed with
fiber based diets to find the optimum pattern of offering feed that will enhance
the production and utilization of caecotropes by the animal. Furthermore, the
supply of small amounts of starch-based feed as a single meal early in the day
[a common practice in many tropical countries] may interrupt the residence time
of caecotropes in the stomach and may also cause disruption of the fermentative
efficiency in the caecum, particularly if the feed contains appreciable
enzyme-resistant starch mainly amylose.
References
Brooks D 1997 Nutrition and Gastrointestinal
Physiology. In: E V Hillyer and K E Quesenberry (ed.) Ferrets, Rabbits and
Rodents- Clinical Medicine and Surgery. p 169. W.B. Saunders Company,
Philadelphia.
BauchopT and Elsden S R 1960
The growth of
microorganisms in relation to their energy supply. Journal of General Microbiology 23:457
Bird S H and Leng R A 1984 Further studies on
the effects of of the presence or absenceof protozoa in the rumen on the live
weight gain and wool growth of sheep.British Journal of Nutrition 52 607
Black J L 2001
Quality Feed Grains - Research
highlights and opportunities. Proceedings of the 10th Australian Barley
Technical Symposium http://www.regional.org.au/au/abts/2001/m3/black.htm
retrieved 19th Oct 2006
Borneman W S, Akin D E and van Eseltine W P 1986
Effect
of phenolic monomers on ruminant bacteria. Applied Environmental
Microbiology.52:1331-1339.
CamaraV M and Prieur D J 1984
Secretion of
colonic isozyme of lysozyme in association with caecotrophy of rabbits. American
Journal of Physiology Gastrointestinal and Liver Physiology 247, G19-G23
Carabano R and J Piquer 1998 The Digestive
System of the Rabbit. In: C de Blas and J Wiseman (ed.) The Nutrition of the
Rabbit. p 1. CABI Publishing, London.
Cheeke P R 1994 Nutrition and Nutritional
Diseases. In: P J Manning,
Chiv Phiny and Lampheuy Kaensombath 2006:
Effect on feed intake and growth of depriving rabbits access to soft faeces.
Livestock Research for Rural Development.Volume 18, Article # 34.
Retrieved, from
http://www.cipav.org.co/lrrd/lrrd18/3/phin18034.htm
Dierick N A, Vervaeke I J, Decuypere J A, Henderickx H K 1990 Bacterial protein synthesis in relation to organic matter
digestion in the hindgut of growing pigs; contribution of hindgut fermentation
to total energy supply and growth performances. Journal Animal Physiology and
Animal Nutrition 63:,220-235
Devendra C and Lewis D 1974 The interaction
between dietary lipids and fibre in the sheep. Animal Production (19), 67-76.
Dobson D E, Prager E M and Wison A C 1984 Stomach lysozymes of ruminants. 1 Distribution and catalytic properties The Journal of Biological Chemistry 259,11607-11616
Dominguez-Bello M G, Pacheco M A, Ruiz M C, Michelangeli, F Leippe M and Pedro M A de 2004 . Resistance of rumen bacteria murein to bovine gastric lysozyme. BioMed Central Ecology 2004 4, 7-13 http://www.biomedcentral.com/1472-6785/4/7 sighted 1/11/2006
Griffiths M and Davies D 1963
The role of the
soft pellets in the production of lactic acid in the rabbit stomach .Journal of
Nutrition 80,171-189
Irwin D M and Wilson A C 1989
Multiple cDNA
sequences and the evolution of bovine stomach lysozyme. Journal of
Biological Chemistry 264(19):11387-11393
Ito Y, Hirashima M Yamada H and Imoto T 1994 Colonic lysozymes of rabbit [Japanese White]: Recent divergence and functional conversion. Journal of Biochemistry 116, 1346-1353
Jenkins J R 1999 Feeding Recommendations for
the House Rabbit. Veterinary Clinics of North America: Exotic Animal Practice.
vol. 2. p 143. W.B. Saunders Company, Philadelphia
KanamuraS, Lehman PG, Kostyuchenke V A,Chipman P
R, Mesyanzhinov V V, Arisaka F and Rossman M G 2002 Structure of the
cell-puncturing device of bacteriophage T4 Nature 415, 553-557
Keller R and Traub N 1974
The
characterization of Bacteroides fragilis bacteriophage recovered from animal
sera: observations on the nature of bacteroides phage carrier cultures.
Journal General Virology 24: 179 - 189.
KlieveA V and Swain R A 1993 Estimation of
ruminal bacteriophage numbers by pulsed-field gel electrophoresis and laser
densitometry. Appl Environ Microbiol. 1993 July; 59(7): 2299-2303
LebasF, Coudert P, Rochambeau H de and Thébault R
G 1997 The Rabbit - Husbandry, Health and Production. FAO Animal
Production and Health Series No. 21
http://www.fao.org/docrep/t1690E/t1690E00.htm
Leng R A and Nolan J V 1984 Nitrogren metabolism in the rumen. Journal Dairy Science 67 1072
MarounekM, Adamec T , Skfiivanova V and Latsik N
I 2002 Fractions of Nitrogen and in
Marounek M, Vovk S J and Skfiivanova V 1995
Distribution of activity of hydrolytic enzymes in the digestive tract of
rabbits. British Journal of Nutrition 73,463-469
McNitt J I, Patton N M, Lukefahr S
D and Cheeke P R 2000 Rabbit Production.
8th Edition, Interstate Publishers, Inc., Danville, IL.
Oba M and Allan M S 2003
Effects of diet
fermentability on efficiency of microbial N production in lactating dairy cows J
Dairy Science 86 195 -207
Orpin C G and Mann E A 1974 The occurrence of bacteriophage in the rumen and their influence on the bacterial population. Experientia 30, 1018-1020
Pok Samkol, Preston T R and Leng R A 2006a
Effect of offering leaves or stems of water spinach on patterns of eating,
consumption of caecotrophs, and excretion of faeces by growing rabbits.
Livestock Research for Rural Development. Volume 18, Article # 78. RetrievedOctober 16, 106, from
http://www.cipav.org.co/lrrd/lrrd18/06/samk18078.htm
Pok Samkol, Preston T R and Ly J 2006b
Effect
of increasing offer level of water spinach (Ipomoea aquatica) on intake, growth
and digestibility coefficients of rabbits. Livestock Research for Rural
Development. Volume 18, Article #25. Retrieved March 7, 2006, from
http://www.cipav.org.co/lrrd/lrrd18/2/samk18025.htm
Preston T R 2006: Forages as protein sources
for pigs in the tropics. Workshop-seminar "Forages for Pigs and Rabbits"
MEKARN-CelAgrid, Phnom Penh, Cambodia, 22-24 August, 2006. Retrieved, from http://www.mekarn.org/proprf/preston
.htm
Preston T R and Leng R A 1987 Matching Ruminant Production Systems with Available Resources in the Tropics and Subtropics. PENAMBUL Books Ltd: Armidale NSW, Australia
Rowe J B and Crosbie G B 1998
The
digestibility of grains of two oats differing in lignin content Australian J
Agricultural Research 39 639-644
Stewart C B, Schilling J W and Wilson A C 1987Adaptive
evolution in the stomach lysozymes of foregut fermenters.
Nature 330(6146):401-404
Stephens A G 1977. Digestibility and
coprophagy in the growing rabbit. Proceedings. of the Nutrition Society 36: 4A.
Stevens C E and Hume I D 1995 Comparative Physiology of the Vertebrate Digestive System. 2nd ed. Cambridge University Press, Cambridge, United Kingdom.
Wallace R J 1983 Digestion of rumen bacteria in vitro British Journal of Nutrition 49, 101-108
Weller and Pilgrim 1974 Passage of protozoa
and volatile fatty acids from the rumen of sheep and from a continuous in vitro
fermentation system. British Journal of Nutrition 323, 341 -351
Wells J E and Russell J B 1996
Why do so many
ruminal bacteria die and lyse so quickly. Journal of Dairy Science 79 1487-1495