Swamp buffalo rumen ecology and its manipulation
M Wanapat
Department of Animal Science
Faculty of Agriculture
Khon Kaen University, Khon Kaen 40002, Thailand
metha@kku1.kku.ac.th
http://web.kku.ac.th/~metha
Abstract
Rumen ecology has been
playing an important role in fermentation process and providing end-products
for ruminants. These studies were
carried out to investigate on rumen factors namely pH, NH3-N, microorganisms
in cattle and swamp buffaloes raised on traditional system. Furthermore, study on diurnal pattern of
rumen fermentation and effect of rumen digesta
transfer from buffalo to cattle was conducted.
Based on these studies, it was found that buffalo and cattle raised
under similar condition exhibited a diverse rumen
ecology. Diurnal fermentation patterns in both cattle and buffaloes were
revealed. It was found that rumen NH3-N
appeared to be a limiting factor. Rumen digesta transfer from buffalo to cattle was achievable.
Monitoring of digesta transfer up to 14 days resulted
in normal rumen ecology as compared to that of original buffalo. However, further research should be
undertaken in these regards in order to improve rumen ecology especially buffalo-based
rumen.
Key words : Swamp buffalo, rumen ecology,
manipulation, digesta transfer
Introduction
The rumen has been well recognized as an essential fermentation vat that is capable of preparing end-products particularly volatile fatty acids (VFAs) and microbial proteins as major energy and protein for the ruminant host. The more efficient the rumen is the better the fermentation end-products being synthesized. In recent years, there has bee increasing research directed to rumen ecology and rumen manipulation (Orskov and Flint, 1989; Martin, 1998; Weimer, 1998). However, most of these papers have dealt with ruminants raised in temperate areas and fed on good-quality roughages and with high levels of concentrate supplementation. However in the tropics, most ruminants have been fed on low-quality roughages, agricultural crop-residues, industrial by-products which basically contained high levels of ligno-cellulosic materials, a low level of fermentable carbohydrate and a low level of good-quality protein. In addition, long dry seasons, a prevailing harsh environment, especially high temperature, low soil fertility and lees feeds available throughout the year, all influence rumen fermentation. Recently, Wanapat (2000) reported on rumen fermentation to increase the efficient use of local feed resources and productivity of ruminants in the topics.
Traditionally, swamp buffaloes (Bubalus bubalis)
have been kept by small-holder farmers in
The objectives of these experiments were to study on the
rumen ecology of buffalo and cattle raised on traditional system and to
identify the rumen fermentation pattern in buffalo and cattle fed on untreated
and urea-treated rice straw.
Materials and methods
Experiment 1
The rumen samples for subsequent study of microbial
populations were obtained from animals kept under traditional village
conditions in the Northeast of Thailand. The experiment was conducted in
September to April when swamp buffalo and crossbred cattle (Brahman x Native)
were both grazing seasonally on available native grasses, rice stubble and rice
straw. No concentrates were given to the animals at this time. A total of 40
animals which were being
kept under similar conditions were identified, brought from a
local marker and slaughtered. The
animals (20 cattle and 20 buffaloes) were of both sexes and were between 2-4
years of age.
Immediately, after slaughtering, samples of fresh digesta (500g) were taken from the rumen of each animal.
Rumen digesta were squeezed through 4 layers of
cheesecloth to ensure a sample which contained microbial population from both
the liquid and solid phases. The subsequent rumen fluid was immediately fixed
with 10% formalin solution ( Galyean
1989). The total direct count of bacteria, protozoa and fungal zoospores were
made using the methods of Galyean (1989) based on the
use of a haemacytometer (Boeco)
and total numbers were studied for bacterial and protozoal
shapes under microscope. Differentiation of rumen fungal zoospores from small protozoa
was based on characteristics having flagellae
while protozoa had ciliates around them. Rumen fluid was diluted using autoclaved distilled water (121oC for 15 minutes) as a medium, by 100, 10 , and
10 times for bacteria, protozoa and fungal zoospores counting using 10x40,
10x10 and 10x40 ocular x objective of haemacytometer,
respectively. For further identifying different shapes of bacteria rumen fluid
was made at 10 times and counted at 10x40 ocular x objective of haemacytometer.
Scanning electron microscopy was used for further determinations of
bacteria, protozoa and fungal zoospores. Samples were fixed in buffered glutaraldehyde dehydrated in a graduated ethanol series and
dried in a criteria-point dryer. They were mounted on aluminum stubs and
sputter coated with gold prior to viewing. The data were subjected to
statistical analysis using students t- test.
Experiment 2
Digestion trial
Six rumen-fistulated buffaloes and
cattle (3 each) were randomly assigned according to a 3 x 3 Latin square design
to receive three roughage sources and the treatments were as follows:
-
T1 = untreated rice straw (URS)
-
T2 = urea-treated (5%) rice straw (UTRS)
-
T3 = URS and UTRS (1:1) (MX)
All animals received the roughage on ad libitum
basis and in addition rice bran was supplemented at 0.5% of body weight. Digestion
trial lasted for 21 days each. Feed
intakes were measured during the first two weeks and followed by a 24-h rumen
fluid sampling for every hour. Samples
were measured for pH immediately and prepared for later analyses of NH3-N,
VFAs, total viable counts of
cellulolytic, proteolytic
and amylolytic bacteria. During the last 5 days the animals were put
onto metabolism crates when 90% of previous feed intakes were given and for
total collection of feed, feces and urine.
Rumen fluid was collected at 0, 4 h-post feeding and
measured for pH immediately and samples were prepared for later analysis of NH3-N
(Bromner and Keeney
1965), volatile fatty acids (VFAs) using HPLC
(Samuel et al 1997), total viable cellulolytic, proteolytic and amylolytic
bacteria were measured using roll tube technique (Hungate 1969).
Digestibilities of nutrients were
calculated. All data were subjected to
ANOVA and treatment means comparisons were conducted by
Digesta transfer study
All rumen fistulated buffaloes and
cattle (3 each) were fed with three kinds of roughage treatments using a 3 x 3
Latin square design: untreated rice straw (URS), urea-treated (5%) rice straw
(UTRS) and URS and UTRS (1:1) (MX). They
were fed for two weeks and then rumen fluid samples were collected at 0 and 4 h
post feeding. Measurements of pH were
taken immediately while other rumen fluid samples were treated and prepared for
later analyses of NH3-N (Bromner and
Keeney 1965), volatile fatty acids (VFAs) using HPLC
as the above. Total viable cellulolytic, proteolytic and amylolytic
bacteria were counted using roll tube technique (Hungate
1969).
After the initial sampling period (3 weeks), the rumen digesta from each buffalo fed on each
respective roughage were transferred to each respective cattle for each
roughage after rumen digesta of the cattle were
removed completely. These transfer were done as quickly as possible to avoid longer exposion of digesta to the
air. After complete transfers, all lids
of fistulae were closed. Samplings of
rumen fluid were take at 0, 4 h post feeding, before transfer, and 7 and 14
days after rumen digesta transfer to be measured for
rumen pH, NH3-N, VFAs and total viable counts of cellulolytic,
proteolytic and amylolytic
bacteria using standard methods as indicated above. All data were subjected to
ANOVA and treatment means were compared using
Results and Discussion:
Experiment 1
The methods used proved successful at estimating the numbers
of micro-organisms in the samples. Data on the numbers of bacteria, protozoa
and fungal zoospores
and shown in Table 1.
|
Table 1.
Numbers of bacteria, protozoa and fungal zoospores in the rumen of cattle and
buffaloes raised under traditional system in the Northeast of Thailand. |
||
|
Item |
Cattle |
Buffaloes |
|
Rumen pH |
6.58 ± 0.12 |
6.60 ± 0.07 |
|
Microbial population, total direct count |
||
|
Bacteria, x 10-8
cells/ ml |
1.36 ± 0.14 |
1.61 ± 0.12 |
|
Coccus,
x 10-5 cells/ml |
1.07 ± 0.70 |
1.28 ± 0.23 |
|
Oval* |
< |
> |
|
Rod* |
< |
> |
|
Protozoa, x 10-5
cells/ml |
3.82 ± 0.88 |
2.15 ± 0.41 |
|
Holotrich |
2.52 ± 0.70 |
1.80 ± 0.36 |
|
Entodiniomorph |
1.30 ± 0.34a |
0.35 ± 0.13b |
|
Fungal zoospore, x 10-6
cells/ml |
3.78 ± 0.78a |
7.30 ± 0.93b |
|
a,b in the same row with different
superscripts differ (P<0.05) |
||
Based on this experiment, it was found that the rumen pH was
similar for both species, but significant differences
were found in the numbers of micro-organisms. There was a trend for a higher
population of bacteria, a lower population of protozoa and significantly more
fungal zoospores in the ruminal fluid of buffaloes as
compared with those of cattle.
The results obtained show microbial counts which are broadly
in agreement with other studies reported by other workers for cattle and
buffalo (Langar et al 1968). Wattanachant
et al (1990) found higher total bacterial count and cellulolytic
bacteria in swamp buffalo than those in cattle. Interestingly, in the present
study where animals were subjected to similar feeding, there were strong trends
for a higher bacteria and lower protozoa counts but significantly more fungal
zoospore counts in buffaloes than those in cattle. As clearly known, rumen
bacteria are far most important among protozoa and fungi, but moreover, their
close association and balances in forming optimal rumen ecology are paramount
in providing useful fermentation end-products for the ruminants.
The presence and role of fungi in the rumen have until
recently been a contentious issue. In earlier years, Orpin
(1975), Akin et al (1983), Akin and Benner (1988) and Ho and Abdullah (1999)
reported the findings of rumen fungi and their roles in degrading ligno-cellulosic materials which stimulated further studies
to be conducted in these regards. Studies by Ho et al (1988)
indicated the presence of fungi in buffalo and in cattle and it was
found that the rate and method of colonization by rumen fungi in swamp buffalo
and native cattle of
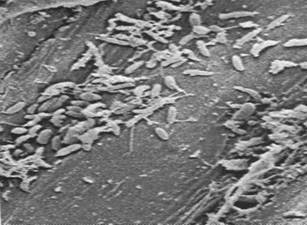
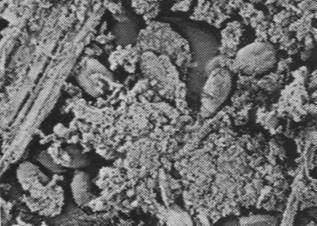
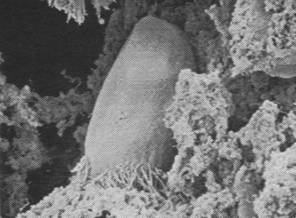
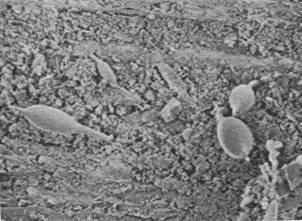
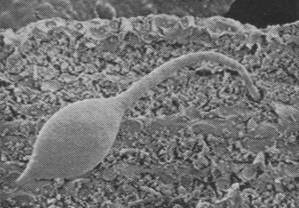
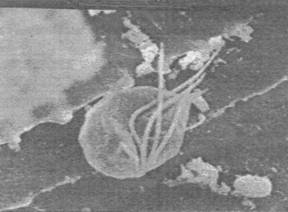
Figures 1, 2, 3, 4, 5, 6 and 7 illustrate pictures of rumen bacteria attachment, protozoa distribution, fungal sporangia, rumen fungus, Anaeromyces sp., having sporangium with acuminate apex and rhizoid forming “appressorium” in swamp buffaloes.
|
|
|
|
|
|
|
|
|
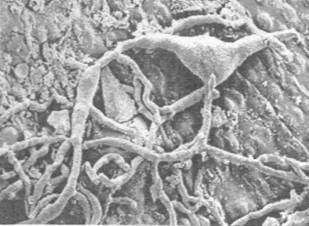
Figure 7. Rumen fungal
sporangium with flagellae, Bar = 16 mm
Experiment 2
The diurnal patterns during 24 h of rumen fermentation characteristics in beef cattle and swamp buffaloes fed on untreated and urea-treated rice straw.
In both cattle and buffaloes, rumen pH and temperature were
maintained constant and the values were 6.5-6.7; 38-39 °C, respectively. However, VFA production patterns were
fluctuating as well as C2 concentration while C3 and C4
were similar which indicated an active role of rumen microbes and on-going
fiber fermentation of cellulolytic bacteria. It was also found that rumen NH3-N
was very consistent and relatively low (<5 mg/100ml) throughout the
period. However, all of the fermentation
aspects except rumen pH and temperature were notably enhanced by feeding
urea-treated rice straw. Rumen fermentation end-products were significantly
different as a result of feeding different types of roughages. As shown in table 2 that rumen NH3-N,
C2, C4 were increased as a result of using urea-treated
rice straw and were also higher in buffalo than in cattle. Ratios of (C2+C4)/C3
and TVFA/NH3-N were also narrower. Based on this study, low rumen NH3-N
could be a limiting factor on rumen fermentation and would ultimately affect on
rumen ecology.
In ruminants fed on low-quality roughages, critical rumen NH3-N
levels for microbial activities were found a 5-20 mg/100ml (Boniface et al
1986; Perdok and Leng 1989).
While Chanthai et al (1987) demonstrated that
rumen NH3-N in cattle and buffaloes fed on untreated rice straw were
less than 2 mg/100ml and were increased to 9 mg/100ml with urea-treated rice
straw. Perdok and Leng (1989) further showed that
higher level of rumen NH3-N (15-30 mg/100ml) improved intake and
digestibility. Increasing rumen NH3-N
level up to 30 mg/100ml significantly decreased C2+C4/C3,
increasing rumen fungal zoospores as well as increasing microbial protein
synthesis (17-47%) (Kanjanapruthipong and Leng 1998). In a most recent experiment in swamp
buffaloes fed on untreated rice straw, Wanapat and Pimpa
(1999) also found similar results that rumen NH3-N levels of
13.6-34.4 mg/100 ml improved rumen fermentation
by increasing digestibility and intake of straw. As rumen NH3-N increased, rumen
bacteria and protozoa, as well as urinary purines
were also increased. It was suggested
that optimum rumen NH3-N level would be higher than 15 mg/100ml.
Nguyen Van Thu and
Effect of buffalo rumen digesta transfer
|
Table 2. Chemical compositions of experimental feeds. |
||||||
|
Item |
DM |
OM |
CP |
NDF |
ADF |
Ash |
|
|
(%) |
% of dry matter |
||||
|
Rice straw (URS) |
92.8 |
88.6 |
3.4 |
76.9 |
48.9 |
11.4 |
|
Urea- treated rice straw (UTRS) |
55.2 |
88.1 |
7.5 |
68.3 |
42.2 |
11.9 |
|
URS + UTRS (1:1) |
79.0 |
88.7 |
5.3 |
73.4 |
46.4 |
11.3 |
|
Extracted rice bran |
90.2 |
84.7 |
14.2 |
12.4 |
4.5 |
15.4 |
|
DM = dry matter, OM = organic matter, CP = crude protein, NDF = neutral detergent fiber, ADF = acid detergent fiber |
||||||
Among diets, urea-treated rice straw (UTRS) digestibility was highest (P<.05) and digestibility of nutrients particularly those of organic matter and crude protein were higher in buffalo than in cattle (Table 3). Several factors have been claimed to attribute to these values.
|
Table 3. The apparent digestibility of feeds and the
effect of digesta transfer on digestibility in
cattle and swamp buffaloes. |
|||||||
|
|
URS |
UTRS |
MX |
SEM |
|||
|
|
C |
B |
C |
B |
C |
B |
|
|
Apparent digestibility, % |
|||||||
|
DM |
50.4a |
54.4a |
63.7b |
63.1b |
55.8ab |
57.9ab |
1.3 |
|
|
51.9a |
57.3ab |
64.3b |
68.4b |
61.9b |
62.2b |
1.2 |
|
CP |
35.4a |
33.7a |
49.7ab |
55.9b |
43.4ab |
41.1ab |
2.5 |
|
NDF |
35.4a |
36.5a |
50.6b |
51.2b |
46.6ab |
47.8ab |
2.9 |
|
ADF |
45.1 |
41.6 |
52.4 |
55.3 |
47.7 |
47.8 |
5.0 |
|
a,b values on
the same row with different superscripts differ (p<0.05) |
|||||||
Intakes of roughages were highest in both cattle and buffaloes fed on UTRS in terms of kg/d, % BW, g/kgW.75. In general, intakes of these roughages before and after buffalo digesta transfer were similar at 7 and 14 days after transfer (Table 4).
|
Table 4. Feed intake in cattle and swamp buffaloes before and 7 and 14 days after transfer of digesta from buffaloes to cattle |
|||||||
|
|
Before |
After 7 d |
After 14 d |
SEM |
|||
|
|
C |
B |
C |
B |
C |
B |
|
|
Total DM intake, kg/d |
|||||||
|
URS |
4.1 |
5.5 |
4.5 |
5.5 |
4.0 |
5.3 |
0.6 |
|
UTRS |
5.1 |
6.5 |
5.3 |
5.7 |
5.3 |
5.5 |
0.5 |
|
MX |
5.2 |
5.6 |
5.7 |
5.9 |
6.0 |
5.8 |
0.5 |
|
% BW |
|
|
|
|
|
|
|
|
URS |
1.2 |
1.4 |
1.2 |
1.2 |
1.4 |
1.3 |
0.1 |
|
UTRS |
1.8 |
1.9 |
1.9 |
2.1 |
2.0 |
1.7 |
0.3 |
|
MX |
1.3 |
1.5 |
1.7 |
1.5 |
1.4 |
1.9 |
0.1 |
|
g/kgW0.75 |
|
|
|
|
|
|
|
|
URS |
72.8 |
81.5 |
73.5 |
83.3 |
77.2 |
84.2 |
8.0 |
|
UTRS |
86.1 |
102 |
87.5 |
92.5 |
97.5 |
92.6 |
10.3 |
|
MX |
93.9 |
84.2 |
93.4 |
88.3 |
96.7 |
84.2 |
3.2 |
|
URS = rice straw,
UTRS = urea-treated rice straw, MX = URS + UTRS (1:1) |
|||||||
Rumen pH in all treatments and animals were similar and were
in normal range of rumen ecology (pH 6.2-6.7). Digesta
transfer did not show any effects (Table 5).
|
Table 5. Effect of digesta
transfer on rumen pH and NH3-N in cattle and swamp buffaloes. |
|||||||
|
|
Before |
After 7 d |
After 14 d |
SEM |
|||
|
|
C |
B |
C |
B |
C |
B |
|
|
Rumen pH |
|||||||
|
0 h post feeding |
|||||||
|
URS |
6.4 |
6.3 |
6.6 |
6.7 |
6.5 |
6.6 |
0.06 |
|
UTRS |
6.4 |
6.1 |
6.4 |
6.3 |
6.6 |
6.6 |
0.08 |
|
MX |
6.2 |
6.4 |
6.4 |
6.8 |
6.5 |
6.4 |
0.08 |
|
4 h post feeding |
|||||||
|
URS |
6.5 |
6.3 |
6.3 |
6.3 |
6.5 |
6.7 |
0.05 |
|
UTRS |
6.4 |
6.2 |
6.1 |
6.1 |
6.5 |
6.6 |
0.07 |
|
MX |
6.6 |
6.5 |
6.2 |
6.3 |
6.5 |
6.5 |
0.06 |
|
NH3-N, mg% |
|||||||
|
0 h post feeding |
|||||||
|
URS |
3.1a |
5.4ab |
3.8a |
6.5b |
5.8b |
6.6b |
0.6 |
|
UTRS |
11.9ab |
12.8ab |
8.9a |
11.7ab |
15.1b |
13.9a |
0.9 |
|
MX |
11.6ab |
9.5ab |
7.9a |
8.9ab |
7.9a |
13.5b |
0.9 |
|
4 h post feeding |
|||||||
|
URS |
6.4 |
6.3 |
6.9 |
7.4 |
5.1 |
6.5 |
0.3 |
|
UTRS |
13.0ab |
10.9ab |
15.2b |
10.0ab |
9.6a |
13.9b |
0.9 |
|
MX |
9.8 |
8.9 |
8.4 |
7.5 |
7.1 |
7.5 |
0.4 |
|
a,b values on
the same row with different superscripts differ (p<0.05) |
|||||||
Rumen NH3-N concentrations were lowest in animals
fed on untreated rice straw (URS) and highest in UTRS fed groups. These NH3-N values remained low in
URS fed group after buffalo digesta transfer of 7 and
14 d, respectively and were lower than those reported as optimum (20-30 mg%) (Boniface et al 1989; Perdok and Leng
1989; Wanapat and Pimpa 1999). Values in
cattle and buffalo fed on UTRS and mixture of URS+UTRS (MX) were found higher
and were maintained after digesta transfer for 14
d. Values after 4 h-post feeding were
slightly increased in some treatments (Table 5).
Total volatile fatty acids (TVFAs)
at 0 h post-feeding were highest in UTRS and in buffaloes, while at 4 h-post feeding
in group fed on UTRS and MX were increased.
After 7 and 14 d transfer, TVFAs of cattle
were comparable to those of buffaloes.
This could be an attributing factor from digesta
transfer. For C2, C3
and C4 all values were similar from, before and after 7, 14 d digesta transfer for both cattle and buffaloes. It is
noticeable that C3 concentrations were relatively high in all fed
groups (Table 6, 7, 8, 9).
|
Table 6. Effect of digesta transfer on total volatile fatty acid (TVFA) in cattle and swamp buffaloes |
|||||||
|
|
Before |
After 7 d |
After 14 d |
SEM |
|||
|
|
C |
B |
C |
B |
C |
B |
|
|
TVFA, mM |
|||||||
|
0 h post feeding |
|||||||
|
URS |
85.9 |
85.7 |
86.7 |
78.4 |
102 |
83.2 |
14.2 |
|
UTRS |
94.6 |
106 |
112 |
116 |
125 |
101 |
11.6 |
|
MX |
91.5 |
104.2 |
110.9 |
99.5 |
99.6 |
85.8 |
9.1 |
|
4 h post feeding |
|||||||
|
URS |
75.7 |
80.5 |
100 |
94.2 |
112 |
85.1 |
10.5 |
|
UTRS |
104 |
120 |
117 |
119 |
104 |
115 |
12.2 |
|
MX |
118 |
107 |
109 |
100 |
104 |
96.5 |
10.0 |
|
URS = rice straw,
UTRS = urea-treated rice straw, MX = URS + UTRS (1:1) |
|||||||
|
Table 7. Effect of digesta
transfer on acetic acid concentration in cattle and swamp buffaloes |
|||||||
|
|
Before |
After 7 d |
After 14 d |
SEM |
|||
|
|
C |
B |
C |
B |
C |
B |
|
|
Acetic acid (C2), mM |
|||||||
|
0 h post feeding |
|||||||
|
URS |
67.2 |
64.6 |
66.2 |
68.4 |
69.3 |
65.7 |
5.4 |
|
UTRS |
70.8b |
68.8ab |
64.2ab |
67.4ab |
62.9a |
70.8b |
2.7 |
|
MX |
65.3ab |
67.9ab |
70.4a |
68.6ab |
62.4b |
68.8ab |
3.0 |
|
4 h post feeding |
|||||||
|
URS |
68.7 |
69.2 |
67.1 |
68.8 |
66.1 |
69.7 |
3.8 |
|
UTRS |
70.5 |
68.9 |
66.6 |
67.6 |
66.8 |
69.5 |
3.7 |
|
MX |
68.7 |
66.9 |
68.7 |
72.7 |
66.6 |
69.2 |
3.6 |
|
a,b values on
the same row with different superscripts differ (p<0.05) |
|||||||
|
Table 8. Effect of digesta
transfer on propionic acid concentration in cattle
and swamp buffaloes |
|||||||
|
|
Before |
After 7 d |
After 14 d |
SEM |
|||
|
|
C |
B |
C |
B |
C |
B |
|
|
Propionic acid (C3), mM |
|||||||
|
0 h post feeding |
|||||||
|
URS |
26.2 |
29.8 |
23.9 |
24.2 |
22.4 |
24.6 |
4.3 |
|
UTRS |
24.0 |
27.6 |
29.3 |
27.8 |
25.4 |
23.4 |
3.5 |
|
MX |
26.9 |
23.2 |
24.8 |
24.6 |
29.5 |
25.8 |
3.5 |
|
4 h post feeding |
|||||||
|
URS |
25.2 |
26.8 |
26.5 |
24.4 |
28.0 |
26.3 |
3.9 |
|
UTRS |
21.9 |
25.7 |
28.1 |
26.6 |
26.3 |
24.1 |
2.9 |
|
MX |
23.5 |
26.4 |
24.4 |
26.6 |
31.1 |
28.8 |
4.3 |
|
URS = rice straw, UTRS = urea-treated rice straw, MX
= URS + UTRS (1:1) |
|||||||
|
|
|||||||
|
|
Before |
After 7 d |
After 14 d |
SEM |
|||
|
|
C |
B |
C |
B |
C |
B |
|
|
Butyric acid (C4), mM |
|||||||
|
0 h post feeding |
|||||||
|
URS |
4.7 |
5.6 |
9.8 |
7.3 |
8.0 |
9.6 |
2.6 |
|
UTRS |
5.2a |
6.9ab |
6.9ab |
4.8a |
11.7b |
6.2a |
2.1 |
|
MX |
7.9 |
8.9 |
8.1 |
10.1 |
8.1 |
5.4 |
2.0 |
|
4 h post feeding |
|||||||
|
URS |
6.0 |
7.3 |
6.3 |
6.8 |
6.0 |
7.3 |
1.7 |
|
UTRS |
7.5 |
5.3 |
6.1 |
5.8 |
6.9 |
8.0 |
1.6 |
|
MX |
7.8 |
6.6 |
6.9 |
4.7 |
5.7 |
5.9 |
2.0 |
|
a,b values on
the same row with different superscripts differ (p<0.05URS = rice straw,
UTRS = urea-treated rice straw, MX = URS + UTRS (1:1) |
|||||||
Effect of digesta transfer on rumen microoganisms
Total viable bacteria counts were found to be similar among
treatments and sampling times. Cellulolytic, proteolytic and amylolytic bacterial counts of cattle were increased after
7, 14 d digesta
transfer. The most pronounced values
were obtained in buffaloes fed on UTRS and particularly
at 7 d after digesta transfer. This could mean that
after removal of digesta, buffalo rumen could still
have functionally higher rumen turn over rate and while in cattle the digesta transfer could be sustainable as seen on 14 d after
transfer (Table 10, 11, 12, 13).
Other means of manipulating the rumen could be used (e.g. feeding
of condensed tannins). Condensed tannins
contained in cassava hay has been shown to improve rumen microorganisms and fermentation
and to enhance rumen by-pass of the dietary protein (Wanapat 2000; Wanapat et
al 1999, 2000a,b)
|
Table 10. Effect of digesta
transfer on total viable bacteria in cattle and swamp buffaloes. |
|||||||
|
|
Before |
After 7 d |
After 14 d |
SEM |
|||
|
|
C |
B |
C |
B |
C |
B |
|
|
Total viable bacteria, 1011CFU/g |
|||||||
|
0 h post feeding |
|||||||
|
URS |
2.1a |
2.9ab |
2.4a |
4.6ab |
3.4ab |
5.7b |
0.9 |
|
UTRS |
2.3a |
3.0ab |
3.4ab |
3.8ab |
4.2ab |
4.8b |
0.7 |
|
MX |
2.6a |
2.8a |
5.0ab |
2.4a |
3.4ab |
5.8b |
0.8 |
|
4 h post feeding |
|||||||
|
URS |
1.2a |
2.8ab |
4.5bc |
4.8bc |
5.6c |
5.1c |
0.6 |
|
UTRS |
2.8a |
3.2a |
5.9b |
5.2ab |
4.7ab |
5.0ab |
0.8 |
|
MX |
3.6 |
3.6 |
4.6 |
4.8 |
3.5 |
5.3 |
1.4 |
|
a,b,c values on
the same row with different superscripts differ (p<0.05) |
|||||||
|
Table 11. Effect of digesta
transfer on cellulolytic bacteria in cattle and
swamp buffaloes. |
|||||||
|
|
Before |
After 7 d |
After 14 d |
SEM |
|||
|
|
C |
B |
C |
B |
C |
B |
|
|
Cellulolytic bacteria, 1010CFU/g |
|||||||
|
0 h post feeding |
|||||||
|
URS |
1.8a |
2.8ab |
3.1ab |
4.2b |
2.2a |
2.5ab |
0.6 |
|
UTRS |
3.4 |
5.9 |
2.7 |
2.7 |
5.1 |
5.7 |
1.9 |
|
MX |
1.9a |
4.1b |
2.6ab |
3.0ab |
4.5b |
2.3a |
0.6 |
|
4 h post feeding |
|||||||
|
URS |
2.9a |
3.5ab |
3.4ab |
5.2b |
3.1a |
3.3ab |
0.6 |
|
UTRS |
4.5a |
10.5b |
5.4ab |
7.1ab |
5.1ab |
4.5a |
1.4 |
|
MX |
2.5 |
5.2 |
3.2 |
6.5 |
3.4 |
2.5 |
1.0 |
|
a,b values on
the same row with different superscripts differ (p<0.05) |
|||||||
|
Table 12. Effect of digesta
transfer on proteolytic bacteria in cattle and
swamp buffaloes. |
|||||||
|
|
Before |
After 7 d |
After 14 d |
SEM |
|||
|
|
C |
B |
C |
B |
C |
B |
|
|
Proteolytic bacteria, 107CFU/g |
|||||||
|
0 h post feeding |
|||||||
|
URS |
1.5a |
2.7a |
2.6a |
7.1b |
4.6ab |
2.5a |
0.6 |
|
UTRS |
2.7a |
4.2ab |
5.2ab |
8.2b |
5.2ab |
5.9ab |
1.1 |
|
MX |
3.8 |
4.2 |
3.6 |
3.9 |
3.6 |
3.7 |
0.9 |
|
4 h post feeding |
|||||||
|
URS |
2.8 |
2.3 |
3.4 |
3.2 |
5.0 |
2.5 |
1.4 |
|
UTRS |
2.4 |
5.7 |
5.2 |
8.8 |
6.6 |
3.5 |
1.8 |
|
MX |
4.4 |
2.5 |
4.6 |
3.2 |
2.8 |
2.4 |
0.6 |
|
a,b values on
the same row with different superscripts differ (p<0.05) |
|||||||
|
Table 13. Effect of digesta
transfer on amylolytic bacteria in cattle and swamp
buffaloes. |
|||||||
|
|
Before |
After 7 d |
After 14 d |
SEM |
|||
|
|
C |
B |
C |
B |
C |
B |
|
|
Amylolytic bacteria, 107CFU/g |
|||||||
|
0 h post feeding |
|||||||
|
URS |
2.6a |
3.0ab |
4.0b |
2.5a |
3.7ab |
4.0b |
0.9 |
|
UTRS |
3.5 |
3.6 |
4.3 |
5.3 |
5.4 |
3.9 |
0.8 |
|
MX |
3.2ab |
2.9a |
3.9ab |
2.7a |
5.8b |
3.3ab |
0.7 |
|
4 h post feeding |
|||||||
|
URS |
3.1 |
3.2 |
3.2 |
2.7 |
4.4 |
3.7 |
0.7 |
|
UTRS |
3.5 |
5.3 |
4.9 |
4.9 |
3.8 |
5.6 |
0.9 |
|
MX |
4.3ab |
5.9ab |
4.9ab |
7.3b |
4.0ab |
3.2a |
1.0 |
|
a,b values on
the same row with different superscripts differ (p<0.05)URS = rice straw,
UTRS = urea-treated rice straw, MX = URS + UTRS (1:1) |
|||||||
Conclusions and recommendations
Experiment 1
Cattle and buffaloes raised under traditional raising system
could result in having different rumen microorganisms. Counts of bacteria and fungal zoospores were
higher and protozoa were lower in the rumen of buffaloes than in cattle. These
could attribute to higher ability to utilize feeds and hence higher
digestibility of feed in buffaloes.
Experiment 2
Rumen NH3-N appeared to be the limiting factor on
untreated straw. UTRS resulted in higher nutritive value than URS and MX. The transfer of rumen digesta
transfer from buffalo to cattle indicated that intake, digestibility and rumen
ecological parameters criteria from the buffaloes could be sustained in cattle
at least for 14 days.
Acknowledgements
The author wishes to express gratitude to the
References
Akin, D.E., G.L. Gordon and J.P. Hogan, 1983. Rumen bacterial and fungal
degradation of Digitaria pentzii grown
with or without sulfur. Appl. Environ. Microbiol.
46:738-748.
Akin, D.E.
and R.Benner. 1988. Degradation of polysaccharides and
lignin by ruminal bacteria and fungi. Appl. Environ. Microbiol. 54:1117-1125.
Boniface, A.N., R.M. Murray and J.P. Hogan. 1986. Optimum level of ammonia in the rumen liquor
of cattle fed tropical pasture hay. In:Proc.
Aust. Soc. Anim. Proc.
16:151-154.
Bromner,
J.M., and D.R. Keeney. 1965. Steam
distillation methods of determination of ammonium, nitrate and nitrite. Anal. Chem. Acta. 32:485.
Chanthai,
S., M. Wanapat and C. Wachirapakorn. 1989.
Rumen ammonia-N and Volatile fatty acid concentrations in cattle and buffalo
given rice straw-based diets. In:Proc.
7th AFAR Int. Workshop. (Eds. R. Dixon), IDPD,
De Haan, H. Steinfeld and H. Blackburn. No date. Livestock and the Environment: Finding a balance. European Commission Directorate-General for Development and Natural
Resources, EU Publication 115 pp.
Devendra, C. 1985. Comparative nitrogen utilization in
Galyean, M. 1989. Laboratory procedure in animal
nutrition research. Department of Animal and Range
Sciences.
Hungate, R.E.
1969. A roll tube method for cultivation of strict
anaerobes. In:Methods in
Microbiology, edited by J.R. Norris and D.W. Ribbons.
Ho, Y.W. and D.J.S. Barr. 1995. Classification of anaerobic gut fungi from herbivores with emphasis on rumen
fungi from
Ho, Y.W. and N. Abdullah. 1999. The role of rumen fungi in fiber digestion.
Asian-Aus. J. Anim. Sci.
12:104-112.
Ho, Y.W., N.Abdullah and S. Jalaludin. 1988.
Colonization of guinea
grass by anaerobic rumen fungi in swamp buffalo and cattle. Anim. Feed Sci. Technol. 22: 161-171.
Kanjanapruthipong
and R.A. Leng. 1998. The effects of dietary urea on microbial populations
in the rumen of sheep. Asian-Aus. J. Anim. Sci. 11:661-667.
Kennedy, P.M. and J.P. Hogan. 1994.
Digestion and metabolism in buffaloes and cattle:are there consistent diffences.
In: Proc. The 1st Asian buffalo Association
Congress. (Eds. M. Wanapat and K. Sommart),
Langar,
P.N., G.S. Sidhu and I.S. Bhatia. 1968. A
study of the microbial population in the ruminal of
buffalo (Bos Bubalis) and
Zebu (Bos indicus) on a
feeding regimen deficient in carbohydrates. Indian. J.
Vet. Sci. 38:333-336.
Nguyen Van Thu and T.R.
Orpin, C.G. 1975. Studies on the rumen flagellate Neocallimastix frontalis. J.
Gen. Microbiol. 91:249-262.
Orskov,
E.R. and H.J. Flint. 1989. Manipulation
of rumen microbes or feed resources as methods of improving feed utilization. In:Proc. The
Biotechnology in Livestock in Developing Countries. (Ed.
A.G. Hunter). Rkitchie of Edinburgh Ltd.
Perdok,
H.B., and R.A. Leng. 1989. Effect of
supplementation with protein meal on the growth of cattle given a basal diet of
untreated ammoniated rice straw. Asian-Aus. J. Anim. Sci. 3:269.
Samuel, M., S. Sagathewan, J. Thomas and G. Mathen. 1997. An HPLC method for estimation of vollatile
fatty acids of ruminal fluid. Indian.
J. Anim. Sci. 67:805.
SAS.
1985. User’s Guide :
Statistics, Version 5 Edition. SAS. Inst.
Wanapat, M. 1989, Comparative aspects of digestive physiology and nutrition in
buffaloes and cattle. In:Proc. Ruminant Physiology and
Nutrition in
Wanapat, M. 2000. Rumen Manipulation to Increase
the Efficient use Local Feed Resources and Productivity of Ruminants in the
Tropics. In:Proc. Of the 9th AAAP Congress. (Eds. G.M. Stone),
Wanapat, M. and O. Pimpa. 1999. Effect of ruminal NH3-N
levels on ruminal fermentation purine
derivatives, digestibility and rice straw intake in swamp buffaloes. Asian-Aus.
J. Anim. Sci. 12:904-907.
Wanapat,M., K Sommart.,C. Wachirapakorn, S. Uriyapongson, and C.Wattanachant. 1994. Recent advances in swamp buffalo nutrition and feeding. In:Proc.The 1st Asian
Wattanachant, C.,M.
Wanapat, S. Sarangbin, S. Chanthai
and C. Wachirap;akorn. 1990. A Comparative stydy on
rumen cellulolytic bacteria in
swamp buffaloes and cattlel. In Proc. 28th
Wanapat, M., O. Pimpa, W. Sripuek, T. Puramongkol, A. Petlum, U. Boontao, C. Wachirapakorn and K. Sommart. 1999. Cassava Hay:an Important On-Farm Feed for Ruminants. In:Tannins in Livestock and Human
Nutrition. (Eds. J.D. Brooker), May
31 June –
Wanapat, M., O. Pimpa, A. Petlum and C. Wachirapakorn. 2000a. Participation scheme of small holder dairy farmers
in the northeast
Wanapat, M., T. Puramongkon
and W. Siphuak. 2000b. Feeding of cassava hay for
lactating cows.
Asian-Aus. J. Anim. Sci.
13:478-482.
Williams, A.G. and G.S. Coleman 1992. The Rumen Protozoa. Springer-Verlag,
Weimer, P.J.
1998. Manipulating ruminal
fermentation: A microbial
ecological perspective. J. Anim. Sci.
76:3114-3122.