Effect of supplementation with sesbania (Sesbania grandiflora) leaves on rumen
parameters, and in vivo,
in sacco and in vitro digestibility in swamp
buffaloes fed rice straw or elephant grass
Nguyen Van Thu
Dept. of Animal Husbandry, Faculty of Agriculture
Cantho University, Vietnam
Tel. 8471-830786, Fax: 8471-830814
nvthu@ctu.edu.vn
Abstract
The ruminal parameters, in vivo, in vitro, in sacco digestibility, and water extractable DM, were measured in buffaloes fed rice straw and elephant grass with supplementation of fresh leaves of Sesbania grandiflora. The design was a 2x2 factorial arrangement with three replications. The first factor of the experiment was feed (rice straw or elephant grass); the second factor was supplementation of 4 kg/day of fresh leaves of Sesbania grandiflora. The buffaloes received the forage at 80% of their requirement and the sesbania leaves were supplemented once in the morning.
Ruminal ammonia concentration, bacteria population and volatile fatty acids were significantly higher for the diets with Sesbania grandiflora supplementation. Supplementation also significantly improved in vivo DM and NDF digestibility but had no effect on in vitro measurement of digestibility nor on "a", "b" and "c" parameters in the in sacco test. However, the effective DM degradability value as measured by the in sacco method was significantly increased by supplementation compared to the control. In vivo DM digestibility was more closely correlated with water extractable DM values than with any of the in vitro analyses.
It is concluded that farmers could supply a supplement of Sesbania grandiflora leaves to buffaloes fed low quality forages to improve their rumen function and production.
Keywords: bacteria, buffalo, in vitro, in sacco and in vivo
digestibility, protozoa, supplementation, Sesbania grandiflora, volatile fatty
acids, water extractable DM
Introduction
Due to the demand for land for crop production in Vietnam, the grassland areas for ruminants has been reduced. This is one of the factors that has contributed to the reduction of the buffalo population reduction in the Mekong delta (Duc 1988). However, buffalo meat has an important role for people in this region. Recently, buffalo production has been favourably considered by farmers as a means of raising their income (Long 2003), but low quality of buffalo feeds, which are mainly crop residues, has dominated in their diets and has limited the buffalo performance. Supplementation with urea-molasses-minerals has improved digestibility and performance (Nguyen Van Thu 2001); however, due to the material cost and transportation, it has been limited in application by farmers in remote villages. On the other hand, plant protein sources as alternative sources of supplements in buffalo diets have been abundant and available in this region such as Sesbania, Leuceana and duckweeds.
Estimation of feed digestibility by the in vivo technique in ruminants has been useful; however, the high cost and time required have been limiting factors to its common use. Alternatively, in vitro and in sacco digestibility methods have been used effectively due to low cost and many more feed samples can be evaluated (Lopez et al 2000). Therefore, this study aimed to investigate whether the rumen environment and feed digestibility could be improved by supplementation of buffalo diets with leaves of Sebania grandflora, using in vitro and in sacco digestibility procedures.
Materials and methods
The study was carried out at the experimental farms of Cantho
University. It was a 2x2 factorial design experiment with 3 replications. The
first factor was feed (rice straw or elephant
grass) and the second factor was supplementation (with and without leaves of
Sesbania grandiflora). The experimental animals were swamp male buffaloes
(420±25 kg live weightfitted with rumen cannulae. The
experimental period was 3 weeks including one week for diet
adaptation. The fresh Sesbania grandiflora leave was
supplemented once at a level of 4 kg per day in the morning. The
experimental animals were fed at 7.00 am and
2.00 pm.
Samples of rumen contents were collected at 3h post-feeding to
measure rumen pH, ammonia N (NH3-N), protozoa and bacteria
populations. Rumen pH was measured by pH meter and NH3-N was
analyzed by the micro Kjeldahl method. For counting protozoa the
preparation of rumen content samples followed the procedure of
Dehority (1984) and a 0.2mm deep chamber under 100 x magnification
was used. Total bacteria populations were counted in a Neubauer
chamber under 1200 x magnification after the preparation of rumen
content samples following the procedure of Warner (1962). Total
VFA were measured by steam distillation following the
procedure described by Barnett and Reid (1957).
Feeds and refusals were collected daily and pooled weekly for
analysis of DM to calculate feed intake. Feeds and samples for
rumen incubation were analyzed for DM, organic matter (OM), crude
protein (CP), neutral detergent fiber (NDF), acid detergent fiber
(ADF), acid detergent lignin (ADL) and ash following the procedure
of AOAC (1990) and Van Soest et al (1991). Animals were weighed on
two consecutive days at the beginning of the experiment and at the
end of each period to calculate the live weight
change.
Feed samples were dried and ground to pass a 1mm sieve for the rumen incubations. In sacco incubation was made at 12, 24, 48, 72 and 96 hours in duplicate to measure feed degradability following the method of Ørskov et al. (1980). Their values were also fitted to the non-linear model DMD= a + b (1- e-ct ) following Ørskov and McDonald (1979). DMD is the dry matter disappeared after time (t), "a" is the intercept of the degradation curve at time zero, "b" is the fraction which degrades with time at rate "c" and "a+b" represent potential degradability. The effective dry matter digestibility (ED) was calculated following Ørskov and McDonald (1979) by ED = a + bc[1-e-(c+k)t]/(c + k), where k = 0,0246 in the case of buffalos (Bartocci et al 1997) and "a", "b" and "c" values fit to the DMD= a + b (1- e-ct ) as above. .
Feed samples were also used for measuring OM degradability in vitro at 12, 24, 48, 72 and 96 h by using rumen fluid as described by Goering and Van Soest (1970). In vivo DM, OM and NDF digestibility were determined by faecal collection for 7 days (Mc Donald 1998). The water extractable DM (WEDM) was determined in duplicate for three representative samples of each of the leaves following the procedure described by Ly and Preston (1997). The samples (1 g) were put in bags (50 x 150 mm) made from nylon filter cloth with a pore size of 45 to 55 microns and thereafter washed at random in one, two, three or four consecutive cycles of 30 min each. The volume of water used in every cycle was in the ratio of 3 litres per bag. After washing, the dry matter in the residue was estimated by microwave radiation to constant weight.
Data were analyzed by the General Linear Model using the software of Minitab (1998) Comparisons between feeds, and supplementation, were made by the Tukey test .
Results and discussion
Feed composition
Rice straw and elephant grass were low in crude protein but a high fiber content, while Sesbania leaves were high in crude protein but low fiber.Thus supplementing Sesbania leaves to the rice straw and elephant grass improved the crude protein content of the diets. Rice straw had a higher lignin content compared to elephant grass.
|
Table 1. Chemical composition of the experimental feeds (as % of DM, except for DM which is on fresh basis) |
|||||||||
|
|
DM |
OM |
Ash |
CP |
CF |
EE |
NDF |
ADF |
Lignin |
|
Rice straw |
79,9 |
83,8 |
16,2 |
4,43 |
32,0 |
2,07 |
69,6 |
41,4 |
13,1 |
|
Elephant grass |
11,5 |
86,3 |
13,7 |
9,86 |
32,1 |
3,76 |
71,5 |
38,3 |
9,09 |
|
Sesbania leaves |
22,4 |
90,5 |
9,50 |
21,8 |
- |
- |
37,3 |
22,5 |
- |
|
Rice straw + Sesbania leaves |
65,8 |
84,2 |
15,8 |
8,82 |
33,6 |
4,08 |
56,1 |
37,2 |
10,8 |
|
E. grass + Sesbania leaves |
12,6 |
85,6 |
14,4 |
12,1 |
31,4 |
4,52 |
60,3 |
36,6 |
10,3 |
|
DM= dry matter, OM= organic matter, CP= crude protein, NDF= Neutral detergent fiber, ADF= acid detergent fiber, CF= crude fiber and EE= Ether extract |
|||||||||
Rumen parameter, VFA production, bacteria and protozoa populations
Ruminal pH was not effected by supplementation (Table 2) and was in the range suitable for the growth and activities of bacteria (Maeng 1998).
|
Table 2. Effect of Sesbania leaves supplementation on Rumen parameters, VFAs production, bacteria and protozoa population of the experimental buffaloes |
|||||||
|
|
Feed(F) |
Supplementation (S) |
Significance level |
||||
|
Ele.grass |
Rice straw |
Suppl. |
No suppl. |
F |
S |
F x S |
|
|
pH |
6,62 |
6,84 |
6,69 |
6,77 |
* |
ns |
ns |
|
Ammonia, mg/100ml |
18,1 |
8,11 |
16,7 |
9,48 |
*** |
*** |
ns |
|
Bacteria count, x 109/ml |
2,83 |
2,02 |
2,76 |
2,09 |
** |
* |
ns |
|
Protozoa count, x 105/ml |
9,94 |
7,43 |
9,84 |
7,54 |
ns |
ns |
ns |
|
VFAs, mM |
113 |
101 |
116 |
98,5 |
ns |
* |
ns |
|
ns non significant difference, * significant difference
(P<0.05). |
|||||||
Ammonia concentration in the rumen was higher when the buffaloes were fed elephant grass, rather than rice straw, and when the diets were supplemented with Sesbania leaves. The rumen ammonia concentration reflected the crude protein levels in the diets and was associated with higher numbers of bacteria. VFA concentration was higher on the supplemented diet; however there was no difference between the grass and rice straw diets. These results are similar to the findings of Bitende and Ledin (1996) and Kaitho et al (1998).
Water extractable DM values and in vivo digestibility
|
Table 3. Effect of the supplement on the washing loss values and digestibility in vivo |
|||||||
|
|
Feed (F) |
Sesbania (S) |
Significance level |
||||
|
Elephant |
Rice straw |
Sesbania |
No sesbania |
F |
S |
F x S |
|
|
Wash loss value at 45min, % |
25,5 |
17,0 |
22,8 |
19,7 |
*** |
* |
ns |
|
Wash loss value at 90min, % |
28,2 |
19,7 |
26,4 |
21,6 |
*** |
* |
ns |
|
DM digestibility, % |
66,6 |
51,1 |
60,5 |
57,2 |
*** |
* |
ns |
|
NDF digestibility, % |
69,4 |
57,1 |
66,2 |
60,3 |
*** |
* |
ns |
|
ADF digestibility, % |
65,6 |
48,6 |
58,9 |
55,3 |
*** |
ns |
ns |
|
ns non significant difference, * Significant at P<0.05, **Significant at P<0.01; *** Significant at P<0.001. |
|||||||
|
Table 4. Effect of sesbania supplementation on coefficients of the in sacco rumen degradation of OM |
|||||||
|
|
Feed (F) |
Sesbania (S) |
Significance level |
||||
|
Elephant grass |
Rice straw |
Sesb-ania |
No sesbania |
F |
S |
F x S |
|
|
a, % |
15,9 |
5,36 |
10,7 |
10,6 |
ns |
ns |
ns |
|
b, % |
54,7 |
57,9 |
54,3 |
58,2 |
ns |
ns |
ns |
|
a+b, % |
70,6 |
63,2 |
65,0 |
68,9 |
* |
ns |
ns |
|
c, %/hr |
3,95 |
2,98 |
3,00 |
3,94 |
ns |
ns |
ns |
|
a intercept of
degradation curve at time zero, b fraction which degrades with time at
rate c and a+b represent potential degradability of y=a+b(1-e-ct).
|
|||||||
|
Table 5. Effect of sesbania supplementation on DM digestibility in sacco at 48 hours |
|||||||
|
|
Feed (F) |
Supplementation (S) |
Significance level |
||||
|
Ele.grass |
Rice straw |
Suppl. |
No suppl. |
F |
S |
F x S |
|
|
a, % |
23,0 |
15,2 |
19,0 |
19,2 |
** |
ns |
ns |
|
b, % |
51,7 |
60,2 |
59,5 |
52,4 |
* |
ns |
* |
|
a+b, % |
74,7 |
75,4 |
78,6 |
71,5 |
ns |
ns |
ns |
|
c, %/hr |
2,82 |
1,75 |
2,86 |
1,71 |
* |
* |
ns |
|
ED, % |
50,0 |
38,1 |
46,6 |
41,5 |
*** |
** |
ns |
|
a intercept of
degradation curve at time zero, b fraction which degrades with time at
rate c and a+b represent potential degradability of y=a+b(1-e-ct);
|
|||||||
 |
 |
|
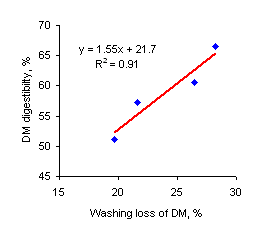
Figure 1. Relationship between washing loss of DM (90 minutes in washing machine) and in vivo DM digestibility |
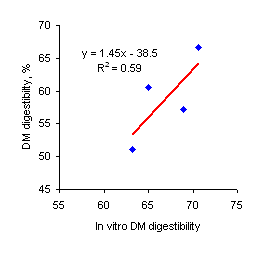
Figure 2. Relationship between in sacco organic matter digestibility (the a+b values in Table 4) and in vivo DM digestibility |
 |
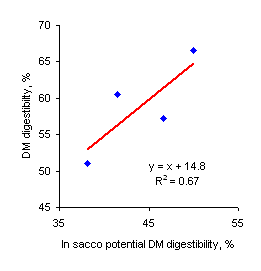 |
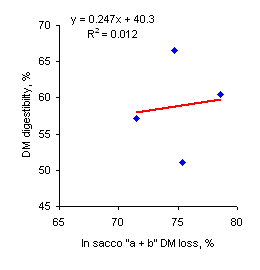
| Figure 3. Relationship between in sacco " a + b" DM loss at 48 hr and in vivo DM digestibility | Figure 4. Relationship between in sacco potential digestibility (ED) and in vivo DM digestibility |
Conclusions and implications
-
Sesbania leaf supplementation improved rumen function parameters and coefficients of digestion in swamp buffaloes fed elephant grass or rice straw as the basal diet.
-
The water extractable DM was a better predictor of in vivo DM digestibility .
-
Farmers could use sesbania leaves to improve performance of their buffaloes due to the supplement being available in their gardens.
Acknowledgements
Financial support of this work was provided by SAREC/Sida under the MEKARN project. The author would like to thank the Department of Animal Husbandry, Faculty of Agriculture, Cantho University, Vietnam for use of their facilities. The author also would like to thank Dr. T.R. Preston and Dr. Brian Ogle and Mr. Börje Ericson for their kind help.
References
AOAC 1990 Official methods of analysis (15th edition). Washington, DC. Volume 1: 69-90.
Barnett A J G and Reid R L 1957 Studies on the production of volatile fatty acids from grass by rumen liquor in an artificial rumen. The volatile fatty acid production from grass. J. Agric. Sci. Cam. 48, 315-321.
Bartocci S, Amici A, Verna
M, Terramoccia S and Martillotti F 1997
Solid and fluid passage rate in buffalo,
cattle and sheep fed diets with different forage to concentrate
ratios. Lives. Prod. Sci. 52, 201-208.
Bitende Stella N and Ledin Inger 1996 Effect of doubling
the amount of low quality grass hay offered and supplementation
with Acacia tortilis fruits or Sesbania sesban leaves, on intake
and digestibility by sheep in Tanzania. Lives. Prod. Sci. 45:
39-48.
Dehority B A 1984 Evaluation of subsampling and fixation procedures used for counting rumen protozoa. Appl. Env, Microbiol. 48, 182-185.
Duc N H 1998 A study of economic and technical criteria of raising swamp buffaloes in the South of Vietnam. BSc thesis (Vitenamese). Cantho University, Vietnam.
Gorering R J and Van Soest P J 1970 Forage fiber analysis (apparatus, reagents and some application ). USDA Agric. Handbook. No. 379, National academic, Washington DC, pp. 1-19.
Kaitho R J, Tegegne A, Umunna N N, Nsahlai I V, Tamminga S, Van Bruchem J and Arts J M 1998 Effect of Leucaena and Sesbania supplementation on body growth and scrotal circumference of Ethiopian highland sheep and goats fed teff straw basal diet. Lives. Prod. Sci. 54: 173-181.
Long D 2002 Buffalo Village. News feature. Tuoi Tre Newspaper (Vietnamese) No. 153/2002 (Aug. 13, 2002). Pp 7.
López S, Dijkstra J and France J 2000 Prediction on energy supply in ruminant, with emphasis on forage. In: Forage Evaluation in Ruminant Nutritive, Givens D I , Owen E , Axford R F E and Omed H M (eds). CAB International, UK: 63-94.
Ly J and Preston T R 1997 An approach to the estimation of washing losses in leaves of tropical trees. Livestock for Rural Development. http://www.cipav.org.co/lrrd/lrrd9/3/ly931.htm
Maeng W J, Kim H J, Chang M B and Park H 1998 Control of rumen fermentation through microbial population change. In prod. Pre-conference Symposia. The 8th World conference on animal production. Seoul. Korea. Pp 511-517.
McDonald P, Edwards R A, Greehalgh J F D and Morgan C A 1998 Digestibility evaluation of foods. In Animal Nutrition. Fifth edition. pp 220-237
Minitab 1998 Minitab reference manual release 12.21. Minitab Inc.
Ørskov E R and McDonald I 1979 The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agr. Sci. Cambridge 92: 499-503.
Ørskov E R, Hovell F D De B and Mould F 1980 The use of nylon bag technique for the evaluation of feedstuffs. Tropical Animal Production 5: 195-213.
Nguyen Van Thu 2001 Effect of urea-molasses-mineral supplementation on in vivo in situ and in vitro feed digestibility of swamp buffaloes. Proceedings Buffalo Workshop, December: http://www.mekarn.org/procbuf/thu.htm.
Van Soest P J, Robertson J B and Lewis B A 1991 Symposium: Carbohydrate methodology, metabolism and nutritional implications in dairy cattle: methods for dietary fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74: 3585-3597.
Warner A C I 1962 Enumeration of rumen Micro-organisms. J. Gen. Microbiology. 1962, 28: 119-128.