|
|
|
|
| Back to content |
Live stock production, climate change and resource depletion |
Rumen fermentation results in the production of a pool of reduced cofactors in the pathways of catabolism of organic matter within rumen microbes. These reduced cofactors are regenerated by 1) synthesis of microbial cells, 2) production of more reduced end products such as propionate and 3) saturation of unsaturated long chain fatty acids but by far the largest proportion of reduced cofactor appear to be regenerated by 4) formation of hydrogen. The concentration of hydrogen in rumen fluid negatively feeds back on the rate of fermentation and microbial growth. The rumen ecosystem has evolved to remove this hydrogen through growth of Archae that obtain energy for their growth by reducing carbon dioxide to methane and water in the rumen.
Ruminal methanogenesis represents a loss of dietary energy to the animal and it is a significant greenhouse gas. Ruminants are credited with a large proportion of the methane accumulating in the world’s atmosphere and a high proportion of the radiative heat forcing of greenhouse gases that have accumulated in the atmosphere. These factors have led to a global search for strategies (including nutritional intervention) to mitigate methane emission from ruminants.
The use of nitrate as a hydrogen sink has been down played, due to the possible toxic effects of nitrite that under some circumstances is formed as an intermediate during the reduction of nitrate to ammonia in the rumen. A few reports have examined the potential of nitrate in vitro as a methane reducing feed additive, which appears to lower methanogenesis consistently.
The rumen ecosystem and the animal need time to adapt to any fermentable N source including urea and nitrate. The requirements for minerals in different dietary conditions appear to be important in determining the microbial consortiums that use these fermentable N sources.
The potential of nitrate conversion ammonia to act as a hydrogen sink in the rumen was reviewed ( Leng 2008)with a clear indication emerging that it is entirely feasible that nitrate could be used as a fermentable N source by ruminants provided the rumen ecosystem was allowed to adapt over a sufficiently long period and provided certain nutritional conditions were met. In particular the availability of sulphur appears to be a crucial issue.
Information derived from other microbial, anaerobic ecosystems showed that in the presence of fermentable organic matter and a source of sulphur and nitrate, nitrate reducing organisms developed that can both reduce nitrate to ammonia and oxidize sulphide to sulphate ( NRSOB) without release of nitrite. At least one prominent rumen organism has this capacity (Wollinella succinogenese) and may be termed an NRSOB.
Studies from the MEKARN group have demonstrated that ruminants fed low protein agro industrial byproducts can utilize nitrate as a fermentable nitrogen source. These groups have been the first to show that nitrate can be fed to ruminants safely under practical conditions and maintain or increase production. Two other groups, with the facilities to measure methane production, have concentrated on the extent to which replacing urea with nitrate salts lowers methane production. Depending on diet and inclusion rate of nitrate, the reduction in methane production has varied from 16-50%. Approximately 1 mole of nitrate in a diet reduces methane production by 10% and there is evidence of an interaction with dietary sulphur levels
The major limitations to progress in using these alternative fermentable N sources that also act as high affinity electron acceptor has been the production of methaemoglobinaemia that results from nitrite generated in the rumen.
The specialized nature and expensive equipment for measuring respired gases from ruminants has also become a major limitation to progress. Considerable advancement appears possible by a new approach where the ratio of methane to carbon dioxide in air receiving the ruminants breath are used to calculate the percentage lowering of methane release from the animal. This approach is possible as it is well demonstrated that where the only intervention is to replace urea with nitrate in a diet the animal’s energy metabolism( carbon dioxide production) is a constant in both control( urea fed) and treatment( nitrate fed ) .
In the present studies a modification of published methods have been used to assess the potential reductions in methane that can be achieved when providing alternative electron acceptor via nitrate in the diet and manipulation of other minerals to promote in particular nitrate reducing sulphide oxidation systems The simplistic and inexpensive requirements make this a useful approach with potential to increase the rate of progress at low cost.
Ruminants have evolved to utilize organic matter, of which most of the digestible energy is present in the structural carbohydrates and to a lesser extent simple sugars or starch. The rumen microbial ecosystem adapts to ferment a wide range of feed materials with the production of microbial biomass and a number of fermentation end products including methane and the volatile organic acids. Microbial cells, synthesized in the rumen, provide the majority of the animal’s requirements for essential amino acids when microbes are washed out of the rumen and are digested and absorbed from the intestines. The supply of microbial protein from the rumen often requires augmentation with dietary bypass protein to ensure optimal quantity and balance of essential amino acids for production from a particular feed. The volatile fatty acids and plant fats (which are relatively unchanged in the rumen) provide the majority of the energy substrates for maintenance and synthesis of new tissue (see Preston and Leng 2009).
On balanced diets, the rumen is a sustainable microbial habitat, consisting of interactive colonies of bacteria, anaerobic fungi and protozoa. The efficiency of this ecosystem depends on maintaining a low redox potential essential for anaerobic fermentation. The reactions involved in the pathways of carbohydrate and protein degradation generate reduced cofactors, which, if not oxidized inhibit fermentation and subsequently lower feed intake. Various strategies have evolved in the rumen to oxidize the reduced cofactors which results in hydrogen release to the medium. Strictly anaerobic Archaea (methanogens) use this hydrogen to reduce carbon dioxide to methane with the generation of ATP for maintenance and cell growth. However, numerous rumen organisms are able to utilize high affinity electron acceptors such as nitrate and sulphate, generating ammonia and hydrogen sulphide respectively.
In ruminants on a range of diets and therefore with diverse rumen microbial ecosystems, it has been clearly demonstrated that the major electron sinks in the rumen milieu include: 1) growth in the microbial biomass, 2) production of propionate, 3) hydrogenation of unsaturated fatty acids and 4) the generation of methane by the Archaea (see Hungate 1965). The latter requires the release/transfer of hydrogen to methanogens by microbes that ferment the components and intermediate products produced from feed materials.
The question posed is whether it is possible to eliminate or significantly reduce methane production by feeding nitrate, to provide an alternative electron acceptor whilst still maintaining the efficiency of the fermentative digestion and therefore the nutritional value of the feed and without compromising the animal’s health.
The potential benefits of nitrate as alternative electron acceptor in the diets of ruminants has received little attention, particularly where it may also supply a high proportion of the total N in a feed and where the ATP produced in reduction of nitrate to ammonia may increase microbial growth efficiency (see Guo et al 2010).
Globally ruminants produce around 80 million tonnes of methane annually, which accounts for about 28% of anthropogenic methane emissions. Reduction in enteric methane production from the large world population of ruminants could significantly reduce global warming. This paper is an update on the report this author produced for the Australian Government’s Climate Control Department (Leng 2008).
A number of research groups have examined the clearance of intra-ruminal nitrate doses in small ruminants (see Figure 1) An examination of this research showed that the rate of uptake (disappearance) of a nitrate dose from rumen fluid was extremely rapid.
|
|
|
|
This rapid initial metabolism of nitrate was previously not recorded because rumen fluid of the injected animals was not sampled until 1 hour after the nitrate solution was placed in the rumen. Making some assumption concerning the size of the rumen fluid pool, the concentration of nitrate assuming instantaneous mixing was calculated (Leng 2008). Within 1 h of administration between 50- 80% of the nitrate dose had disappeared from the rumen contents in studies reported in the literature (see Figure 1). The conclusion is that within 1 hour of injection of a nitrate load, the rate of removal of nitrate from rumen fluid slowed to a rate approximating 0.2 of its initial rate of removal.
The abrupt change in the rate of nitrate clearance is perhaps indicative of an exhaustion of a critical nutrient involved in nitrate metabolism which may be the result of a deficiency of sulphide in the rumen fluid pool. This is argued later in the review.
In studies by Sar et al (2004) where sheep were given an intra-ruminal load of nitrate there was apparently only a small effect of nitrate loading on ruminal fluid ammonia levels suggesting that nitrate was being reduced by bacteria to ammonia intracellular and not being released into rumen fluid unlike what happens when urea is hydrolyzed in the rumen. The other aspects of these loading studies are that nitrite builds up in the rumen and peaks long after the rapid removal phase of nitrate metabolism has occurred (Figure 2). This can be explained if the there is a rapid metabolism of nitrate to ammonia which is then slowed by a deficient of critical nutrients causing nitrite to be spilled into the medium.
|
|
|
|
Figure 2. The theoretical dilution of CrEDTA in the rumen in relation to the changes in nitrite and nitrate levels in the rumen of a sheep following administration of 25 g Na-nitrate intra-ruminally (after Sar et al 2004a). |
|
In studies with cows fed a mixture of hay and concentrates in two meals per day (Kemp et al 1977), the change in nitrite and nitrate concentration in rumen fluid are shown in Figure 3 after the animals had ingested a meal of high nitrate forage (over 45 min) in the morning. Prior to this experiment the animals had been on this feeding regime for 18 d. Although the cattle were accustomed to a daily dose of nitrate, the nitrate and nitrite concentrations appearing in rumen fluid were transient and both were cleared within 2-3 h (see Figure 3). Again there appears to be a rapid clearance of nitrate and a lag phase before nitrite spilling commences.
The opportunity to use nitrate as a fermentable nitrogen source resides in being able to ameliorate or prevent the production of nitrite because of the adverse effects of nitrite, which when absorbed creates methaemoglobinaemia. The relationships between nitrite and nitrate toxicity have been the subject of much research and often heated debate.
|
|
|
Figure 3. Changes in nitrate and nitrite in rumen fluid of a cow following ingestion of hay containing 82g of K-nitrate (Kemp et al 1977) |
|
|
|
Figure 4. Relationship between the peak concentration of nitrite in rumen fluid and methaemoglobin in the blood of cows fed various rations with added K-nitrate including hay, turnip, freshly mown grass or concentrate (Kemp et al 1977). |
A comparison using cattle of feeding nitrate sprayed onto hay as against drenching a similar load of nitrate, indicated that the rate of intake of nitrate is an important issue as it could be expected that nitrite production in the rumen will depend on the concentration of nitrate in the rumen at any one time. In feeding trials with dairy heifers, the lethal dose of nitrate in feed was found to be 3 times that injected directly into the rumen or about 1g/kg live weight (Crawford et al 1966).
Geurink et al (1989) pointed out that the rate of diffusion of nitrate from the feed in the rumen was an important factor affecting how much methaemoglobin accumulated in blood. The rate of diffusion of nitrate from a fodder together with the rate of consumption of the forage interacts to affect the level of nitrite appearing in rumen fluid and therefore the amount of nitrite absorbed and hence the percentage methaemoglobin produced.
|
|
|
Figure 5. Changes in methaemoglobin in blood of cattle with increasing amounts of nitrate entering the rumen (after Crawford et al 1966). |
From Figure 5 it seems reasonable to suggest that cattle can be fed up to a level of 20-25g NO3 /100kg body weight with minimum nitrite production in the rumen even in non-adapted cattle.
In a 200kg beef steer consuming approximately 6kg dry matter say of 50% digestibility (3kg of fermentable organic matter) the addition of 50g of nitrate could supply the fermentable N for at least 0.5 k g fermentable organic matters and lower methane production of the order of 10%.
A large number of microbial species found in the rumen have the capacity to reduce nitrate or nitrite to ammonia (see Cheng and Phillips 1988; Cheng et al 1985). These organisms are quickly transferred between animals and their population densities increase with nitrate in a diet (Cheng et al 1985; Alaboudi and Jones 1985). As sheep are adjusted to dietary nitrate, the rates of both nitrate and nitrite reduction in rumen fluid increased from 3-10 fold (Alaboudi and Jones 1985; Allison and Reddy 1984) (see also Figure 6).
|
|
|
Figure 6. Effect of level of K-nitrate fed to sheep on the nitrate and nitrite disappearance from strained rumen fluid incubated in vitro (Alaboudi and Jones 1985). |
The increase is associated with marked increase in populations of nitrate-reducing bacteria (Allison and Reddy 1984). Other studies of sheep by Allison and Reddy (1984) showed that introducing nitrate for the first time into the rumen, increased the rate of nitrate reduction in strained rumen fluid, in just 4 h, by 15 fold from the low level of activity prior to nitrate introduction. This increase from very low to substantial levels is further increased on a daily basis with time from nitrate’s first inclusion in the diet (Allison and Reddy 1984: Aloubadi and Jones 1985). Thus the accumulation of both nitrate and nitrite in the rumen depends on how long the animal has been accustomed to nitrate in the feed. Surprisingly nitrate clearance, as used in the standard approach initiated by Lewis (1961) has not been repeated with animals acclimated to nitrate.
In 40kg sheep given a diet of lucerne/ground maize, nitrate and nitrite levels in rumen fluid were undetectable but on day 1 of inclusion of nitrate (0.17g of NO3- /kg body weight) at 1 h post feeding, nitrate and nitrite concentration in rumen fluid were 1.7 and 0.26 m-mol/litre. respectively. Within 6 days the rumen microbes had adapted and nitrate and nitrite were so rapidly metabolized that their concentrations in rumen fluid were undetectable 1h post feeding (Allison and Reddy 1984).
In a study with sheep, Alaboudi and Jones (1985) increased dietary KNO3 concentration every 2 weeks (from 0 to 2.5g/kg body weight in 0.5g/kg body weight steps). The rate of removal of added nitrate or nitrite in strained rumen fluid taken from those sheep at the end of each adjustment period was measured in vitro. Three flasks of strained rumen fluid were incubated with both substrate and residual nitrate, or nitrite was measured after 1, 2 and 3h and the rate of disappearance calculated from the slope of plots relating residual nitrate or nitrite to incubation time (see Figure 6).
Nitrate added to the diet of sheep rapidly promotes multiplication of microbes that utilize nitrate as a nitrogen source, or what seems to be more likely induces or increases the activity of nitrate reductase systems in microbes that are already present in the rumen. This increase (up to15 fold) occurs rapidly over the first few hours (Allison and Reddy (1984)) and the capacity to handle nitrate increases with time of exposure and amount of nitrate fed (a further 3-10 fold). The capacity to use nitrate returned to pre-treatment levels within 3 weeks of removal of nitrate from the diet of sheep (Aloubadi and Jones 1985).
There is a great deal of apprehension about feeding nitrate to ruminants which has largely stemmed from research that attempted to explain the toxicity syndrome in ruminants. Methaemoglobinaemia represents a significant risk that is probably manageable in the same way as the risk of urea poisoning is avoided, mainly by slow adaptation to a dietary source of nitrate. Marais et al (1988) have pointed out that nitrite and nitrate may more subtly affect animal production through detrimental indirect effects on rumen organisms, particularly through toxic effects on cellulolytic organisms (Hall et al 1960) that may lower the apparent digestibility of forage or pure cellulose in vitro. Recent research (Trinh Phuc Hao et al 2009) clearly indicates little or no harmful effects in the rumen since nitrate supplementation of a sugar cane-based diet to young goats promoted similar growth ratesv as with urea (see Figure 7) and there are now many research papers that lead to the same conclusion.
|
|
Figure 7. Growth curves of goats fed calcium nitrate or urea as NPN sources in a basal diet of chopped sugar cane and fresh cassava foliage (Nguyen Ngoc Anh et al 2010) |
However, there are many studies that report toxicities with nitrate in the diet. The confusion in the literature is probably owing to the different diets and possibly effects of other requirements in nitrate metabolism.. Molybdenum has key effects on nitrate metabolism (Tillman 1965) and sulphur is also implicated by the work of Sokolowski (1969). The effects of nitrate metabolism in reducing the production of hydrogen sulphide in polluted water and in oil well water purged by salt water provide possible leads to the interactive effects of sulphate and nitrate metabolism in anaerobic systems. Addition of nitrate to sewage as a remedial to the smell of bad eggs (H2S production) has been known for more than a century and is still used in for instance some of the sewers in Paris.
That there are interactions between nitrate and sulphur metabolism in anaerobic ecosystems is clear ( ). In situations where oil organics are fermented, nitrate application apparently blocks hydrogen sulphide production (Greene et al 2003) and balance studies in sheep have shown that nitrate feeding decreased sulphur retention (Sokolowski et al 1969).
Anaerobic fungi in the rumen require a source of reduced
sulphur for growth (Gordon and Phillips 1998). These organisms are essential for
adequate comminution of highly lignified plant materials in the rumen and a
study of the effects of nitrate supplementation on their sulphur requirements is
a priority research area.
In ruminants, forage is initially mechanically shredded in the process of harvesting, chewing and rumination. The particle size of feed in the rumen is further reduced by the growth of the radii of anaerobic fungi through the plant tissues. The mechanical and fungal initiated reduction in particle size increases the surface area of substrate and exposes digestible polysaccharides allowing a high density of microbes, mainly bacteria, to attach and solubilize structural carbohydrates, proteins and other components of the feed with the production of volatile fatty acids (VFA). The energy released in conversion of feed to VFA is partially used in microbial growth. A critical requirement in a continuous fermentation is the oxidation of reduced cofactors that are generated in the conversion of organic matter (largely carbohydrate) via the Embden Meyerhof pathway and additional pathways to VFA (see Figure 8). In the rumen, the reduced cofactors must be regenerated by electron transfer to acceptors other than oxygen and the major electron sink in the rumen is methane that is produced by the reduction of carbon dioxide; however, rumen micro-organisms can use both sulphate and nitrate as alternative electron acceptors. The generalized outline of methane production from carbohydrate (glucose) is shown in Figure 8.).
|
|
|
|
Nitrate is a potent inhibitor of methanogenesis in all systems, from fermentative digestion in the rumen to secondary fermentation in a wide range of systems from anaerobic biodigestors to sediments (Hungate 1965; Allison et al 1981; Akunna et al 1994). Respiratory conversion of nitrate to ammonia by anaerobic organisms is highly competitive as an electron sink consuming 8 electrons in the process and out-competing methanogens for electrons in agreement with the free energy change in the reactions which are -598 kJ for the reduction of nitrate to ammonium and -131 kJ for the reduction of carbon dioxide to methane (see Allison and Reddy 1984; Allison et al 1981).
The predominant pathway of nitrate metabolism in the rumen is uncertain but has always
been assumed or even asserted to be dissimilatory nitrate reduction to ammonia, the overall two step reduction of nitrate shown below,
NO3- + 2H+ à H2O+ NO2--------------------------------------------------------------------------------------- Equation 1
NO2- + 6H+ à H2O+ NH3-------------------------------------------------------------------------------------- Equation 2
Organisms capable of nitrite ammonification usually have the ability to reduce nitrate to
Nitrite in dissimilatory metabolism (Simon 2002), nitrite being a suitable electron acceptor for anaerobic respiration. Formate and hydrogen are the common electron donors in assimilatory nitrite ammonification.
These substrates are oxidized according to equations 3 and 4 below: The sulphate reducing organisms (SRB) are an exception with the majority of the Desulfovibrio species using nitrite but not nitrate (Mitchell et al 1986). Recently in anoxic ecosystems with high sulphur content sulphide has been shown to function as an electron donor for respiratory nitrite ammonification in nitrate reducing sulphide oxidizing bacteria (NR-SOB) that oxidize hydrogen sulphide directly (Hubert and Voordouw 2007) (equation 5).
3HCO-2 +NO-2+5H+ à 3 CO2+NH+4 +2H2O------------------------------------------------ Equation 3
3H2 + NO-2 +2 H+ à NH+4 +2H2O-------------------------------------------------------------------- Equation 4
3HS- + NO2- +5H+ à 3S0 + NH+4 +2H2O--------------------------------------------------- Equation 5
The reaction in equation 5 suggests that nitrate or nitrite uptake could be stimulated by a source of sulphide produced locally. As most organisms that reduce sulphate are also capable of reducing nitrate or nitrite, the feeding of nitrate and sulphate is likely to have important interactions and, in some environments, sulphate reducing organisms are inhibited by nitrite.
Sokolowski et al (1960) showed that sheep can tolerate levels of KNO3 in their diets, that had previously been considered lethal, and grow normally. In subsequent publications, Sokolowski et al (1969) found that adding 3.2% KNO3 to a concentrate-based diet, with or without added sulphur, lowered the overall growth rates of lambs offered the diet over 48d (see Table 1). However, there appears to have been no acclimation period, so the slight drop in live weight gain where nitrate was included in the diet could have been a result of a reduced initial growth when the animals were acclimating to the nitrate. Carver and Pfander (1973) found that 21 d was needed to enable sheep on similar diets to acclimate to KNO3. In the second experiment, lambs (26 kg) were offered the same diet but restricted to an intake of 1200g/d (Sokolowski et al 1969). . The addition of 3.2% nitrate had little effect on N retention but N balance increased significantly when sulphur was also included in the diet (Table 1 and Figure 9). .
|
Table 1. The effects of adding KNO3 and /or sulphur to a concentrate based, well balanced diet for sheep (weight 35-39kg). The diet contained maize (30%), soybean meal (10%), corn starch (21-24%), ground corn cob (25%), molasses (6%) and maize oil (2%) and the sheep were also given recommended amounts of minerals and vitamins. There were 5 lambs per treatment. |
||||
|
Supplement |
+0.4% sulphur |
+3.2% K-nitrate |
+3.2%K-nitrate +0.4% sulphur |
+3.2%K-nitrate+0.8% sulphur |
|
Dietary crude protein (%) |
8.6 |
10.5 |
10.5 |
10.5 |
|
Dietary sulphur (%) |
0.52 |
1.2 |
0.52 |
0.92 |
|
Daily gain (g/day) |
220 |
170 |
170 |
200 |
|
Feed intake(g/day) |
1.4 |
1.53 |
1.45 |
1.51 |
|
Feed conversion ratio (g/g) |
7.0 |
8.8 |
8.6 |
7.5 |
|
Sulphur balance (g/day) |
0.67 |
-0.8 |
1.22 |
2.86 |
|
N balance (g N) |
3.95(1.12)* |
3.35(1.58) |
5.60(3.18) |
3.62(4.94) |
|
Wool growth (g per 100cm2/day) |
2.3 |
2.6 |
2.6 |
2.5 |
|
*SD of the mean |
||||
Nitrate appears to have a major effect on sulphur metabolism in the rumen. In this study, when nitrate was added to the diet, the apparent sulphur balance in the animal decreased from +0.67 to -0.8 g/day (Table 1). This could have resulted from high nitrate in the diet inhibiting SRB in the rumen (see Nikolic et al 1984).
Hydrogen sulphide is produced from S-amino acids in protein and inorganic sulphur in the rumen and passes into the gas space from which it is lost by eructation. Some of the eructated gases are re-inhaled and enter the lungs and most of the sulphide is absorbed into blood (Dougherty et al 1965), then oxidized to sulphate and excreted in the urine.
An important conclusion from the study of Sokolowski et al (1969) was that nitrate had no ill-effects in lambs on diets that were apparently already adequate in fermentable N. However, with sulphur supplementation, nitrate appeared to enhance the animal’s protein nutrition. Wool growth in sheep is highly correlated with the amount of protein absorbed from the intestines. In the sheep in the studies of Sokolowski et al 1969) there was a tendency for clean wool production to increase with the addition of nitrate (Table 1). In addition the digestibility of nitrogen was highest when both nitrate and sulphur were present in the diet.
|
|
|
Figure 9. N-balance (g/N/day) in lambs given a high concentrate diet supplemented with nitrate or nitrate and sulphur (after Sokolowski et al 1969). |
Carver and Pfander (1974) examined the interaction of urea and nitrate in a diet of Timothy hay and molasses (92:8) supplemented with soybean meal that should have been adequate in fermentable N. Supplementing such a diet with 1, 2, 4 or 5% of either urea or KNO3 had no serious ill-effects on the animals, despite increasing urea and ammonia concentrations in blood. No nitrite toxicities were observed during nitrate supplementation. These workers tentatively suggested that when urea and K-nitrate were supplemented to the basal diet, the rumen micro-organisms utilized the nitrate first, thus decreasing the rate of urea hydrolysis.
In young goats, increasing the nitrate concentrations in a low protein feed in steps every 7 d converted a negative N balance at low concentrations (0.3 and 0.6%) to a positive and increasing N balance at 1.2, 2.4 and 4.8% of the diet (see Figure 10), clearly demonstrating that nitrate was efficiently used as a fermentable nitrogen source for microbial growth in the rumen (Trinh Phuc Hao et al 2009). Growth rates or N balance were the same in goats fed the same basal diet but including 1% of body weight as tree foliage when either nitrate or urea was the major fermentable nitrogen source (unpublished). In the most recent research the diet was purposely supplemented with a source of sulphur at the upper levels of recommended inclusion (0.4% of diet)
|
|
|
Figure 10. Changes in N balance during introduction of K-nitrate to goats receiving a basal diet of rice straw and molasses (Trinh Phuc Hao et al 2009) |
The effects of supplementing cattle fed a basal diet of caustic soda-treated rice straw clearly demonstrates that sodium nitrate or ammonium nitrate produce the same benefits as urea as the fermentable nitrogen source in these diets (Table 2).
|
Table 2. Mean values for feed intake, DM apparent digestibility and change in live weight of cattle fed NaOH-treated rice straw and fermentable rumen N from sodium nitrate (SN), ammonium nitrate (AN) or urea (Ngoc Huyen Le Thi et al 2010) |
||||||
|
|
SN |
AN |
Urea |
SEM |
Prob |
|
|
Live weight, kg |
|
|
|
|
||
|
Initial |
190 |
195 |
194 |
2.33 |
0.39 |
|
|
Final |
196 |
201 |
200 |
2.1 |
0.39 |
|
|
Daily gain |
0.476 |
0.453 |
0.429 |
0.024 |
0.50 |
|
|
DM intake, kg/d |
5.57 |
5.52 |
5.43 |
0.11 |
0.70 |
|
|
DM digestibility,% |
63.5 |
62.6 |
61.7 |
0.72 |
0.220 |
|
In a recent study (Nolan et al 2010), sheep were fed oat hay and either potassium nitrate or urea (5.4 g N/kg hay), first in metabolism cages and then in respiration chambers. Methane production was reduced by feeding nitrate instead of urea but there were no effects on feed intake, DM digestibility or microbial protein synthesis (Table 3).
|
Table 3. Effect of N from potassium nitrate or urea on methane production from sheep fed oat hay (adapted from Nolan et al 2010). |
|||
|
|
Urea |
4% KNO3 |
Probability |
|
DM intake, g/day |
863 |
870 |
NS |
|
DM digestibility, % |
59.4 |
56.8 |
NS |
|
Methane, liters/kg DMI |
29.8 |
22.9 |
0.04 |
.
The conclusion from the feeding trials is that nitrate can be used as a source of fermentable N in the rumen and, provided the animal is acclimated to nitrate, there will be no ill effects and possibly improved efficiency of microbial growth. It has been hypothesized that nitrate utilization increases the requirements for dietary sulphur (see Leng 2008) to maintain nitrate conversion to ammonia without spilling nitrite. This is supported by the results of Van Zijderveld et al (2010a), where both nitrate and sulphate additions to the diet of sheep produced the greatest reduction in methane production without any signs of methaemoglobinaemia, It is also supported by the feeding trials in Vietnam (Nguyen Ngoc Anh et al 2010) where sulphur levels were deliberately high and close to the upper recommended inclusion levels. However, this aspect requires considerably more research where nitrate and sulphate levels are varied. Therefore substantial mitigation of methane production may be anticipated where nitrate replaces urea in low protein diets given to ruminants but again research is needed to test this concept.
This has been discussed earlier but the hypothesis has now more support so the main points are given below (see Leng 2008)
The hypothesised pathways occurring in the rumen are shown in Figure 11. Heterotrophic NRB reduce nitrate to ammonia using either formate or hydrogen as the electron source. NRB however out-compete the SRB for the same electron donors and may reduce sulphide production The NR-SOB reduce nitrate through nitrite and couple reduction of nitrite to the oxidation of sulphide to sulphate. This can also reduce the availability of hydrogen sulphide. When nitrate is adequate but sulphide is limiting in the medium the NR-SOB obtain maintenance energy requirements (or ATP) by converting nitrate to nitrite with 0.25 of the electron requirements and nitrite is spilled into the medium. Nitrite concentrations increase in the incubation medium and further inhibits SRB activity ( Based on a similar system proposed for the anaerobic oil well water with added nitrate to prevent souring( production of hydrogen sulphide) (see Hubert and Voordouw 2007).
|
|
|
Figure 11. Hypothesized impact of increased sulphur metabolism in association with nitrate. as the fermentable nitrogen source. (A) Sulphide produced by SRB activity can be recycled to sulphate or sulphur by NR-SOB reducing nitrate to ammonia (B) hNRB compete with SRB for organic electron donors, such as lactate, excluding sulphide production by SRB and possibly lowering their capacity to switch to nitrite reduction. Many SRB and hNRB oxidize lactate incompletely to acetate and CO2 as shown. The overall reactions in panels A and B are the same: the oxidation of lactate with nitrate (from Hubert and Voordouw 2007). SRB,NRB NR-SOB are present in the rumen and the end-products could be produced without denitrification because of the short turnover time of rumen contents |
Until recently there had been no attempts to study dietary nitrate utilization in ruminants in relation to the effects on methanogenesis, where nitrate represents the sole or major source of dietary fermentable nitrogen. Feeding trials with young animals appear to confirm the use of nitrate as a supplement to low protein diets with no side effects (Trinh Phuc Hao et al 2009; Ngoc Huyen Le Thi 2010; Nguyen Ngoc Anh et al 2010).
|
|
|
Figure 12. The effects on methane production of administration of a nitrate load into the rumen of fed sheep un-acclimated to nitrate in their diet (Sar et al 2004a). |
Takahashi and Young (1991) showed that nitrate inhibited methanogenesis in sheep in vivo when 25-30 g sodium nitrate was added via the rumen in un-acclimated animals that were also fed diets high in fermentable nitrogen. In a similar study by Sar et al (2004a) shown in Figure 12, sheep were fed half their ration at zero hour and nitrate or water (control) was administered at 30min after the animals were fed. Inhibition of methane production appeared to be delayed by some 30min Thus there is strong evidence that nitrate addition to the diet of ruminants severely inhibits methane production and more so in the animal adapted to nitrate (Allison et al 1981; Allison and Reddy 1984)).
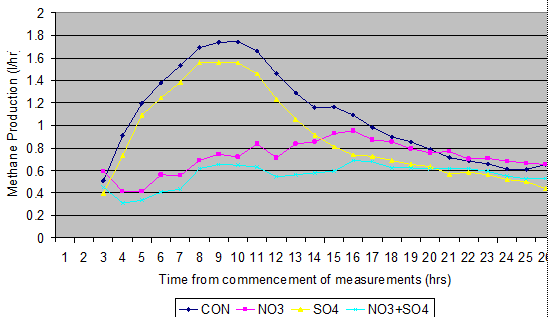
Van Zijderveld et al (2010a) have made the most comprehensive study of the effects of nitrate and sulphur on enteric methane production in sheep In their studies, lambs were gradually introduced to nitrate (3.4% of diet DM) with or without sulphate (3.5 % in diet DM) or urea with or without sulphate (1.5%+3.4% of DM) in a maize silage based diet over 4 weeks and CH4-production was subsequently determined in respiration chambers. CH4-production was significantly decreased by both supplements (nitrate: - 32%, sulphate: -16% and nitrate + sulphate: -47% relative to the urea control). The decrease in CH4-production as a result of nitrate feeding was most pronounced in the period directly after feeding, whereas the CH4-reduction associated with sulphate feeding was observed during the entire day (Figure 13). CH4 suppressing effects of nitrate and sulphate were independent and additive. Nitrate in the diet induced only slightly elevated blood levels of methemoglobin. When nitrate was fed in combination with sulphate, methemoglobin was not detected .
 |
|
Figure 13. The effects over a day on the patterns of methane production in sheep fed maize silage and concentrate The feed was given at 7 30 am .(Van Zijderveld et al 2010a) |
Similar results were shown by Nolan et al (2010).In the latter studies, sheep were acclimated to one of three diets consisting of chaffed oat hay supplemented with 0%, 2% or 4% KNO3 and made iso-nitrogenous by the addition of urea (4 sheep/diet). Nitrate supplementation did not raise blood methaemoglobin, reduce DM intake or affect whole tract or ruminal (in situ) DM digestibility. Nitrate did cause changes in rumen fermentation consistent with its acting as a high-affinity hydrogen acceptor, i.e. a reduced propionate molar percentage in rumen VFA, increased molar acetate: propionate ratio and a lower methane yield per kilogram of DM ingested (for the diet with 4% KNO3, methane was reduced by 23% compared to the sheep fed urea only). The data confirm the capacity of dietary nitrate to reduce enteric methane production but these researchers found large between-animal variation in methane production and better technology or the use of larger numbers of animals will be required to quantify the effect with confidence.
More recently research has been reported at the Greenhouse Gases and Animal Agriculture conference (2010) showing that both beef cattle and lactating dairy cows may utilize nitrate as a high-affinity hydrogen acceptor and also as a fermentable non protein nitrogen source to support rumen fermentation with significant mitigation of enteric methane production.
Hulshot et al (2010) working in Brazil with Bos indicus cattle fed a total mixed ration of sugar cane and concentrates (60:40) supplemented with iso-nitrogenous amounts of either urea (1.2% of DM) or nitrate (2.2% of DM) showed that methane emissions (measured using the SF6 inert gas as a marker) were 32% lower on the nitrate diet with little other effects. Unlike the case for many feed additives, where the rumen system adjusts and with time the differences between treated and untreated declines, with nitrate the effects are persistent.
In high yielding dairy cows fed a maize silage based diet and producing on average 33kg milk/day (Van Zijderveld et al 2010b), it was found by calorimetry that replacing 1.5% urea with 2.2% nitrate reduced methane production by 16% and that the effects, following adaptation to nitrate (over 4 weeks), were persistent over a 4 month period. There was a minor increase in methaemoglobin levels in blood but the animals displayed no clinical signs of methaemoglobinaemia.
Any method of measurement of methane production in ruminants has serious draw backs, for instance the need for calorimetric equipment or complex instrumentation for measurement of inert gases. The large variations in individual estimates, particularly by the inert gas procedures (Pinares-Patino and Clark 2008). together with the need for specialized skills has prevented large scale research, particularly in the developing countries where research priorities for animal production are quite different to the systems in industrial countries. Furthermore, for any National mitigation scheme the wide range in types of diets fed in developing countries is a further complication. Also there are some serious doubts about the accuracy of measurements. In studies where the reduction in methane production is the target a more simplified approach may be taken.
Recently Madsen et al (2100) proposed a greatly simplified approach to quantifying methane production from ruminant animals. Under most research requirements it is a measure of the percent reduction of methane to a particular input that is required particularly when feed additives are being assessed for their methane-inhibiting abilities. This particularly applies to the addition of high affinity electron acceptors to provide an electron sink other then methane. Nitrate has a dual role of being able to accept electrons more efficiently then carbon dioxide. Nitrate is also a source of non protein nitrogen which provides N to the ammonia pool in the rumen (see Leng 2008), a prerequisite for the supplementation of ruminants fed crop by-products low in crude protein.
New developments initiated in the MEKARN program in SE Asia (http://www.mekarn.org/workshops/pakse/abstracts.htm have opened up the methane mitigation research for rapid progress with ruminants fed diets that are more likely to be adopted by small-scale farmers. A modification of the method advocated by Madsen et al (2010) is being used to assess the potential reductions in methane that can be achieved when providing alternative electron acceptors via nitrate in the diet. The simplistic and inexpensive requirements make this a useful approach with potential to increase the rate of progress at low cost. Madsen et al (2010) adopt the concept that provided carbon dioxide production by ruminants can be estimated, or calculated, then a measurement of the methane to carbon dioxide ratio in gas samples allows a reasonably accurate method for predicting methane produced by the animal. In these authors’ views the carbon dioxide can be calculated from metabolisable energy values either from past research or from measurements of heat production.
The concept that is being developed through the MEKARN research builds on that of Madsen et al (2010.). In studies where nitrate has replaced urea as a fermentable N source in ruminant diets it has been well demonstrated that energy metabolism is not perturbed and the animals have consumed the same quantity of dry matter and have grown at the same rate (Nguyen Ngoc Anh et al 2010). It can therefore be assumed that the animals would have the same carbon dioxide entry rate. If this is the case, then the ratio of methane to carbon dioxide in breath or even in air in an animal house can be used to determine the percentage reduction in methane where urea in the diet is replaced by nitrate. These can be instantaneous samples captured from the actual breath or by enclosing the animal or its head in a closed space (Photo 1). The gases that contain the breath of the animal can then be collected and analyzed.
|
|
|
Photo 1. Wooden crates enclosed in plastic are used to house the animals (goats) during the 5 minute period of measurement using the GASMET infra-red analyser (Gasmet Company, Finland) |
If the methane production rate is (A) and the carbon dioxide entry rate (B) is unchanged, and the ratio of methane to CO2 is ( R), then the following equations apply
For the urea supplemented animal
AU(urea)=B x R1
AN(nitrate =B x R2
Then Methane reduction= B(R1-R2)/BR1 x100
The approach is simple. The requirements are portable instruments for measuring methane and carbon dioxide and methods to collect a gas sample that contains sufficient respired gases from the animal. This can be done where animals are concentrated in rooms that are partially airtight or by enclosing an animal in a sealed container (Photo 1) or by direct collection of gas from the head of the animal (Photo 2) . The mechanisms for collection can be as innovative as necessary. The requirement is that the study should be adequately replicated and that the animals have the same energy metabolism or are adjusted to the same basal diet.
Van Zijderveld et al (2010a) measured both carbon dioxide and methane production in sheep fed nitrate, or sulphate, or nitrate plus sulphate added to a control diet based on corn silage (Table 2).Methane production was directly affected by the supplements and there was a small significant difference in carbon dioxide production between treatments . Never the less the reduction in methane production calculated from the methane to carbon dioxide ratio (calculated from the daily production of both gases) compared favorably to the actual measurement of the reduction in methane production (Table 2; Figure 15). If gas had been withdrawn from the chambers at a constant rate, then the ratio of the gases would clearly have supplied a good estimate of the percent reduction in methane.
|
Table 2. The relation between the reduction in methane production as calculated from the ratio of methane to carbon dioxide and as measured in calorimeters from repeated gas sampling over 24 hours. |
|||
|
Treatment |
Methane /carbon dioxide ratio |
Calculated reduction of methane, % |
Measured reduction in methane,% |
|
Control |
0.058 |
0 |
0 |
|
+Nitrate |
0.049 |
13 |
15.5 |
|
+Sulphate |
0.042 |
32 |
27.5 |
|
+Nitrate+Sulphate |
0.030 |
47 |
48.3 |
There seems to be no reason why the methane carbon dioxide ratio cannot be used to define the extent of methane mitigation either at a point in time or by obtaining a sample of gas from a chamber continuously or by frequent sampling of any gas phase that receives the animals’ breath. To quantify the degree of methane mitigation it would be necessary to either use metabolisable energy values from the literature or make individual measurements by the now traditional procedures.
Iv Sophea et al (2010) trained young goats to quietly breathe into a plastic bag for gas collection (Photo2).
 |
| Photo 2. Taking samples of breath from goats |
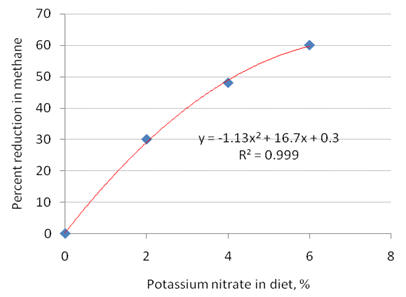
Respired gases were collected at 4 30 pm over 3min. This was 1 h after a meal of rice straw and mimosa foliage which had been provided at 8 am had been consumed. The fermentable N source was either urea or nitrate to provide iso-nitrogenous quantities. Nitrate, as potassium salt was varied from 0 to 8% of the ration as shown in Figure 13. The calculated methane reduction from the methane carbon dioxide ratio is shown in Figure 14.
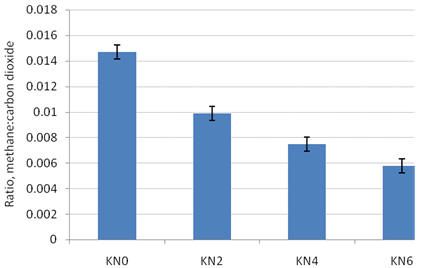 |
| Figure 13. Mean values for ratio of methane to carbon dioxide in goats fed a basal diet of rice straw supplemented with foliage of Mimosa pigra (KN is level of potassium nitrate as % of diet DM replacing iso-nitrogenous quantities of urea) |
 |
|
Figure 14. The reduction of methane release in goats fed rice straw and mimosa foliage calculated from the gas ratios of methane to carbon dioxide (after correction for background levels) (Iv Sophea et al 2010) |
Akunna J C, Bizeau C and Moletta R 1994 Nitrate reduction by anaerobic sludge using glucose at various nitrate concentrations: ammonification, denitrification and methanogenic activities. Environ Technol 15:41-49
Alaboudi R and Jones GA 1985 Effects of acclimation to high nitrate intake on some rumen fermentation parameters in sheep. Can J Anim Sci 65:841-849
Allison M J and Reddy CA 1984 Adaptations of gastrointestinal bacteria in response to changes in dietary oxalate and nitrate. In “Current Perspectives in Microbial Ecology”, Proceedings of the 3rd International Symposium on Microbial Ecology, 7-12 August 1983. Klug MJ, Reddy CA (eds) Amer Soc Microbiol Washington DC
Allison M J, Reddy C A and Cook H M 1981 The effects of nitrate and nitrite on VFA and CH 4 production by rumen microbes. J Anim Sci 53 (Suppl. 1) 283
-416
Cheng K J and Phillippe R C 1988 Identification of rumen bacteria that anaerobically degrade nitrite. Can J Microbiol 34:1099–1102
Cheng K L, Phillippe R C, Kozub G C, Majak W and Costerton J W 1985 Induction of nitrate and nitrite metabolism in bovine rumen fluid and the transfer of this capacity to untreated animals. Can J Anim Sc 65:647-652
Crawford R F, Kennedy W K and Davison K L 1966 Factors influencing the toxicity of forages that contain nitrate when fed to cattle. Cornell Vet 56:3-17
Dougherty R W, Mullenax C H and Allison M J 1965 Physiological phenomena associated with eructation in ruminants. Page 159 In “Physiology of Digestion in the Ruminant”. Dougherty R W (ed) Butterworths Inc, Washington De
Gordon G L R and Phillips M W 1998. The role of anaerobic gut fungi in ruminants. Nutr Res Rev 11:133–168
Guo W S, Schaefer D M, Guo X X, Ren L P and Meng Q X 2009 Use of nitrate-nitrogen as a sole dietary nitrogen source to inhibit ruminal methanogenesis and to improve microbial nitrogen synthesis in vitro. Asian-Australian Journal of Animal Science 22, 542-549
Greene E A, Hubert C, Nemati M, Jenneman G and Voordouw G 2003 Nitrite reductase activity of sulphate–reducing organisms prevents the inhibition by nitrite reducing, sulphide oxidizing bacteria. Environ Microbiol 5:607-617
Hall O G, Gaddy C D and Hobbs C S 1960
Effect of nitrates and
nitrite upon forage utilization by rumen microorganisms in vitro and upon ration
digestibility by lambs. J Anim Sci 19:1305(Abstr)
Ho Quang Do, Preston T R and Leng R A 2008 unpublished results
Hubert C and Voordouw G 2007 Oil field souring control by nitrate-reducing Sulphurospirillium spp. that outcompete sulphate-reducing bacteria for organic electron donors. Appl Environ Microb 73: 2644-2652
Hulshof R B A A, Berndt J J, Demarchi A, Gerrits W J J and Perdok H.B 2010 Dietary nitrate supplementation reduces methane emission in beef cattle fed sugarcane-based diets Greenhouse Gas Conference Banff Canada , page 81 http://www.ggaa2010.org/pdfs/Proceedings_Abstracts.pdf
Hungate R E 1965 The rumen and its microbes. Academic Press, New York
Leng R A 2008 The potential of feeding nitrate to reduce enteric methane production in ruminants. Report to Department of Climate Change, Commonwealth Government,
Canberra. 82 pp. www.penambulbooks.com
Lewis D 1951 The metabolism if nitrate and nitrite by the sheep. 1. Reduction of nitrate in the rumen of the sheep. Biochemistry Journal 48:175-179
Kemp A, Geurink J H, Haalstra R T and Malestein A 1977 Nitrate poisoning in cattle. 2. Changes in nitrate in rumen fluid and methaemoglobin formation in blood after high nitrate intake. Neth J Agri Sci 25:51-62
Madsen J , Bjerg B S, Hvelplund T M, Weisbjerg R and Lund P 2010 Methane and carbon dioxide ratio in excreted air for quantification of the methane production from ruminants, Livest. Sci. (2010, doi:10.1016/j.livsci.2010.01.001
Marais J P, Therion J J, Mackie R I, Kistner A and Dennison C 1988 Effect of nitrate and its reduction products on the growth and activity of the rumen microbial population Brit J Nutr 59:301-313
Mitchell G J, Jones J G and Cole G A 1986 Distribution and regulation of nitrate and nitrite reduction by Desulfovibrio and Desulfotomaculum species. Arch Microbiol 144:35-40
Nikolic J A, Jovanovic M and Andric R 1984 Some effects of nitrate on microbial metabolism in rumen contents. Acta Veterinaria, Beograd 34, 3-14.
Nolan J V 1999 Stoichiometry of rumen fermentation and gas production. In: ‘Meeting the Kyoto Target, Implications for the Australian Livestock Industries”, Reyenga PJ, SM (eds) pp. 21-28: Bureau of Rural Sciences
Nolan J V, Hegarty R S, Hegarty J, Godwin I R and Woodgate R 2010 Effects of dietary nitrate on rumen fermentation, methane production and digesta kinetics in sheep. Animal Production Science 50(8) 801–806 doi:10.1071/AN09211
Ngoc Huyen Le Thi, Do H Q, Preston T R and Leng R A 2010 Nitrate as fermentable nitrogen supplement to reduce rumen methane production. Livestock Research for Rural Development. Volume 22, Article #146. http://www.lrrd.org/lrrd22/8/huye22146.htm
Nguyen Ngoc Anh, Khuc Thi Hue, Duong Nguyen Khang and Preston T R 2010 Effect of calcium nitrate as NPN source on growth performance and methane emissions of goats fed sugar cane supplemented with cassava foliage. Proceedings MEKARN Conference 2010 Live stock production, climate change and resource depletion. http://www.mekarn.org/workshops/pakse/abstracts/anh_grrc.htm
Preston T R and Leng R A 2009
Matching Livestock Systems to
Available Resources in the Tropics and Sub Tropics. Penambul Books,
Armidale, Australia.
Web edition.
http://utafoundation.org/P&L/preston&leng.htm
Sar C, Santoso B, Mwenya B, Gamo Y, Kobayashi T, Morikawa R, Kimura K, Mizukoshi H and Takahashi J 2004b Manipulation of rumen methanogenesis by the combination of nitrate with β1-4 galacto-oligosaccharides or nisin in sheep. Anim Feed Sci Technol115:129–142
Sokolowski J H, Hatfield E E and Garrigus U S 1969 Effects of inorganic sulphur on KNOa utilization by lambs. J Anim Sci 28:391-396
Tillman A D, Sheriha G M and Sirny R J 1965 Nitrate reduction studies with sheep. J Animal Sci 24:1140-1146
Trinh Phuc Hao, Ho Quang Do, Preston T R and Leng R A 2009 Nitrate as a fermentable nitrogen supplement for goats fed forage based diets low in true protein. Livestock Research for Rural Development. Volume 21, Article #10. http://www.lrrd.org/lrrd21/1/trin21010.htm
Van Zijderveld S.M, Gerrits W.J J, Apajalahti J A, Newbold
J R, Dijkstra J, Leng R A and Perdok H B 2010a Nitrate and sulfate:
effective alternative hydrogen sinks for mitigation of ruminal methane
production in sheep. Journal of Dairy Science. Volume 93, 5856-5866
http://download.journals.elsevierhealth.com/pdfs/journals/0022-0302/PIIS0022030210006387.pdf
Van Zijderveld S M, Dijkstra J, Gerrits W J J, Newbold J R and Perdok H B 2010b Dietary nitrate persistently reduces enteric methane production in lactating dairy cows. In Greenhouse gases and animal agriculture conference. October 3-8, 2010 Banff, Canada, T119, page 127 http://www.ggaa2010.org/pdfs/Proceedings_Abstracts.pdf