| Use of Cassava as Animal Feed |
Effects of cassava leaf meal on the
rumen environment of local yellow cattle
fed urea-treated rice straw
Duong Nguyen Khang and Hans
Wiktorsson*
College of Agriculture and Forestry, National University of Ho Chi Minh
City, VietNam.
*Swedish University of
Agricultural Sciences, Department of Animal Nutrition and Management,
PO Box 7024, 750 07, Uppsala, Sweden
Abstract
An experiment was conducted as a Latin square design with four rumen-fistulated local Yellow cattle with a mean live weight of 230 kg. The treatments were: (CLM0) urea-treated rice straw ad libitum plus 1 kg/d cassava root meal (basal diet), (CLM500) basal diet plus 500 g/d cassava leaf meal, (CLM1000) basal diet plus 1000 g/d cassava leaf meal, and (CLM1500) basal diet plus 1500 g/d cassava leaf meal.
Total dry matter intake
increased with increasing level of cassava leaf meal. The ruminal ammonia
concentration and populations of protozoa and bacteria tended to increase with
increase in CLM supplementationon treatment. The in sacco degradability of cassava leaf
meal was high (85 % of
the DM had disappeared after 24 h of incubation) and of urea-treated rice straw was 64% after 72 h of incubation.
There was a slight tendency for in sacco degradability of rice
straw to increase with CLM supplementation.
Key
words: Cattle, cassava leaf meal, rice straw, intake, rumen
environment, degradability
Introduction
In Southeast Asia rice
straw is the predominant dry season feed for ruminants, despite its low
nutritive value (Wanapat 1984). It is deficient in readily fermentable energy,
nitrogen, minerals and vitamins, and cannot provide for optimum microbial
growth in the rumen or tissue development of the host. As a result, growth
rates and milk production are generally low and often only about 10 % of the
genetic potential of the animal (Leng 1995).
Chemical and physical
treatment of rice straw has been widely practiced as a method of improving
intake and digestibility (Sundstol and Coxworth,1984). Ammoniation using urea
has received major attention as an appropriate system for developing countries
(Owen and Jayasuriya 1989). Further improvement in performance may be achieved
by supplementing treated rice straw with fresh or dried forage. A good
candidate for supplementation is cassava forage, which contains more than 20 %
crude protein (Reed et al 1982), in a form which by-passes the rumen, since it
is bound in a tannin-protein complex.
Cassava forage has been shown to be an excellent
source of protein, as a direct supplement or in concentrate mixtures (Wanapat
1995). In the Dominican Republic, fresh cassava leaves as the only source of
forage in a diet of molasses-urea, supported good growth rates (>800 g/day)
in fattening cattle (Ffoulkes et al 1978; Ffoulkes and Preston 1978; Ffoulkes
and Preston 1979). The integral cassava plant has been used for dairy cow
feeding as a supplement to pasture (Garcia et al 1994, Garcia and Herrera
1998).
The hypothesis behind this study is that cassava
leaf meal, fed together with urea treated rice straw, will provide sufficient protein and energy for
the growth of young cattle.
The objectives of the study were:
- to examine the effects of different levels of cassava leaf meal on
ruminal ammonia concentration, ruminal pH and ruminal microflora
population in heifers fed diets based on urea-treated rice straw and
cassava root meal;
- to study the rate and extent of rumen degradability of the feeds on the different rations.
Materials and methods
Location
The experiment was carried out at the experimental farm of
the College of Agriculture and Forestry, Ho Chi Minh City, Vietnam. The mean
air temperature was 28.2 °C and the mean relative humidity 76.5 %.
Animals
Four local Yellow cattle
(20 - 24 months of age and 230 kg average live weight) were fitted with
permanent rumen cannula 3 months before the commencement of the experiment.
Housing
The animals were placed in individual stalls in a barn with
open sides. Clean, fresh water was available ad libitum during the whole
experiment.
Experimental design
The treatments were arranged in a 4*4 Latin square. Each
treatment period lasted for 30 days. The first two weeks of each period were
for adaptation of the heifers and of the rumen microflora to the new diets.
Data on daily feed intake were taken during 7 days of the third week. Feed
samples for analysis were taken before feeding during the last 3 days of the
same week, in sacco degradability was measured during the following 3 days of
the fourth week, and rumen samples were taken during the last 2 days of each
period.
Diet and treatments
The dietary treatments
were:0, 500, 1000 and 1500 g
DM/day of cassava leaf meal (CLM0, CLM500, CLM1000 and CLM1500). The cassava
leaves were collected at the same time from one field after harvesting the
roots. The leaves were air dried and ground. The animals had access to the
feeds for the whole day.
The basal diet consisted of urea-treated rice straw offered ad libitum and supplied once daily at about 07:30 h, together with a supplement (1 kg DM/animal/day) which consisted of cassava root meal and 20 g of a mixture of salt and minerals. The straw was treated with 50 g urea per 1000 g DM of straw, wrapped in an airtight plastic film and stored for 3 weeks before feeding. All the cassava root meal was bought on the market on one occasion.
Measurements
All the feeds were weighed before feeding and supplied
separately to the heifers. Refused feeds were weighed each morning during 7
days of the third week. The feeds were also sampled at these occasions and
analyzed for calculation of daily dry matter and organic matter intake,
according to the procedures of AOAC (1990).
Feed samples were taken for analyses of nitrogen, ether
extract, neutral detergent fiber, and acid detergent fiber (Table 1). The
nitrogen and ether extract of the feed samples
were determined according to the procedures of AOAC (1990). The neutral
detergent fiber (NDF) and acid detergent fiber (ADF) concentrations of feed
samples were determined according to the procedure of Van Soest et al (1991). In
sacco degradation of feed samples was determined during 72 h of each
period. The bags were 60 x 120 mm and made from nylon filter cloth with a pore
size of 28 microns (Saatifil PES 28/17), according to the procedure described
by Orskov et al (1980). The sun-dried samples were milled in a grinder (1 mm)
and weighed amounts (1.5 g) put in nylon bags. The bags were attached to
plastic tubes and incubated in the rumen for 6, 12, 24, 48 and 72 hours. After
incubation, the bags were washed by hand under running tap water until the
water ran clear, and dried in a microwave oven to a constant weight.
During 2 days (day 28 and day 29) of each 30 day period,
ruminal fluid samples were collected
before feeding in the morning and then at intervals of 2 hours over an 8-hour
period through a probe placed in a caudal position in the ventral part of the
rumen. The protozoa population in the rumen fluid was estimated by diluting 8
ml of ruminal fluid with 16 ml of formaldehyde-saline solution (37 %
formaldehyde with saline solution 1:9) and counting the protozoa under
light-microscopy (100x magnification) using a 0.2 mm deep Dollfus counting
chamber. Four fields in the counting chamber were filled and protozoa counted,
according to the method described by Jouany and Senaud (1979) and Dehority
(1993).
A sample of rumen fluid was diluted 1:3 in formol saline
solution and again diluted to 1:3 in formol saline solution (1 part of formol
37 % and 9 parts of saline 0.9 % solution). The fixed samples were stained with
2.5 mg
of 4’, 6 - diamidino - 2 phenylindole (DAPI; Sigma) per ml for 30
min. Each sample was filtered onto 0.2-mm-pore-size nucleopore
filters. Cells were counted at a magnification of x1000 with a Nikon
epifluorescence microscope equipped with a 100-W Hg lamp and an UV filter set
(Navas et al 1993; Joux and LeBaron 1997).
The rumen fluid pH was determined immediately after
collection, by pH meter. The concentration of ammonia nitrogen in the rumen fluid
(NH3-N) was determined by diluting 15 ml of ruminal fluid with 5
drops of concentrated H2SO4 and distilling and titrating
the released ammonia by the standard Kjeldahl procedure (AOAC 1990).
Data were analyzed by ANOVA using the General Linear Model and
Pairwise comparison in Minitab Statistical Software version 12.21.
Results and discussion
Chemical composition
|
Table
1. Chemical composition of feed
ingredients used in the four periods (on DM basis except for DM% which is “as
fed”) |
||||||
|
|
DM |
OM |
N*6.25 |
EE |
NDF |
ADF |
|
URS |
69.1 |
79.6 |
9.67 |
1.14 |
40.7 |
62.1 |
|
CLM |
91.7 |
91.4 |
22.5 |
7.57 |
18.8 |
25.6 |
|
CRM |
92.1 |
92.3 |
1.16 |
2.29 |
2.34 |
3.51 |
|
URS=
urea treated rice straw, CLM= cassava leaf meal, CRM=cassava root meal. |
||||||
Feed intake
The intake of straw and the total dry matter intake showed a
continuous increase with increasing level of cassava leaf supplementation
(Table 2). Cassava leaf meal accounted for 18, 17, 10 and 0% of the total
dietary DM intake for treatments CLM1500
through CLM0. These results differ from those of Queiroz et al (1998a),
who found no differences in dry matter intake of urea-treated corn stover, with
or without supplementation of cassava hay, but are supported by the findings of
Mom Seng et al (2001) who reported increased total DM intake by supplementing
untreated rice straw with fresh cassava foliage. The differences in results
between the studies are probably due to the differences in the chemical and
nutritional quality and physical structure of the supplemented cassava feeds.
The cassava leaf meal in the present study and the fresh cassava foliage used
by Mom Seng et al (2001) contained 22 to 22 % crude protein, as compared
to 14.1% crude protein in the cassava hay used by Queiroz et al (1998a).
|
Table 2. Daily intakes (kg DM) of feeds by local yellow
cattle |
||||||||
|
|
CLM0 |
CLM500 |
CLM1000 |
CLM1500 |
Prob. |
|||
|
URS |
3.62a |
3.67a |
3.97b |
4.32c |
0.02 |
|||
|
CLM |
0a |
0.440b |
0. 940c |
1.075d |
0.001 |
|||
|
CRM |
677 |
505 |
660 |
640 |
0.12 |
|||
|
Total |
4.30a |
4.61a |
5.57b |
6.04b |
0.001 |
|||
Effects of cassava leaf meal levels on rumen pH and ruminal NH3-N
The ruminal pH and NH3-N concentrations
tended to increase with increasing amounts of cassava leaf meal (Table 3). Urea
entering the rumen is hydrolyzed by microbial ureases to CO2 and
ammonia (Van Soest 1982). Later, ammonia is combined with hydrogen ions in the
rumen fluid to form ammonia ions. This process depends on ruminal pH
(Kajanapruthipong and Leng 1998). Ammonia moves readily across membranes as
compared to ammonium ions, and appears to be more readily absorbed (Mooney and
O’Donovan 1970). Chalmers et al (1971) found that when the pH in ruminal fluid
is below 6.9, ammonia concentrations in both peritoneal liquor and jugular
blood decrease, whereas the ammonia concentration in ruminal fluid remains
constant.
|
Table 3. Changes in
ruminal pH and NH3-N, number of protozoa and bacterial population in rumen fluid of local yellow cattle
before and 2h after feeding |
||||||
|
|
CLM0 |
CLM500 |
CLM1000 |
CLM1500 |
SEM |
Prob. |
|
pH |
|
|
|
|
|
|
|
Before |
6.74 |
6.65 |
6.46 |
7.43 |
0.31 |
0.24 |
|
2 h after |
6.22 |
6.61 |
6.33 |
6.49 |
0.21 |
0.62 |
|
NH3-N, mg/100ml |
||||||
|
Before |
7.93a |
7.58a |
8.22b |
11.45c |
0.72 |
0.02 |
|
2 h after |
15.5 |
12.6 |
17.3 |
24.7 |
4.6 |
0.37 |
|
Protozoa
(x10-6/ml) |
|
|
||||
|
Before |
2.97 |
3.10 |
4.72 |
4.63 |
0.63 |
0.18 |
|
2 h after |
1.71 |
2.15 |
3.82 |
4.36 |
1.05 |
1.31 |
|
Bacteria
(x10-9/ml) |
|
|
|
|||
|
Before |
1.09 |
1.38 |
1.75 |
1.70 |
0.36 |
0.57 |
|
2 h after |
0.99 |
1.00 |
1.22 |
1.20 |
0.25 |
0.86 |
Effects of cassava leaf meal levels on microbial populations in the rumen
Protozoal and bacterial populations in the rumen tended to
increase with the increasing level of cassava leaf meal, especially the protozoa
population. This result contrast with the finding of Mon Seng et al (2001) that
cassava forage supplementation rice straw had no effect on the population of
either large or small protozoa.
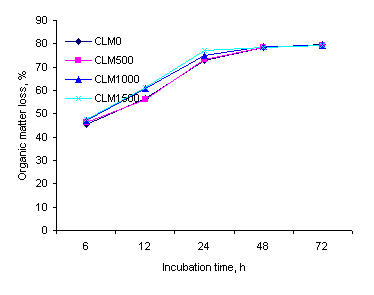
Effects of cassava leaf meal levels on in sacco degradability of feeds in the rumen
The degradation rate of cassava leaf meal was high, 75% of
the organic matter having disappeared after 24 h of incubation. Degradability
of the urea treated rice straw was slower with the maximum at about 65% of the
organic matter after 72 h of incubation (Figures 1 and 2). Increasing levels of
cassava leaf meal tended to increase the rate of degradability but the effects
were rather small. These results are similar to those of Wanapat et al (1997), who found that the degradability of cassava
leaf meal after 72 h of incubation was 78.7 %.
|
Figure 1:
Effect of cassava leaf meal supplementation on loss of organic matter from urea-treated rice straw in the rumen |
Figure 2:
Effect of cassava leaf meal supplementation on loss of organic matter from cassava leaf meal in the rumen |
Acknowledgments
The authors are grateful to the Swedish
International Development Authority (SIDA/SAREC) and the International
Foundation for Science (IFS) for equipment and funding for this study.
References
AOAC 1990 Official methods of analysis of the Association of Official
Analytical Chemists (15th Ed), Washington, DC. 1: 69-90.
Chalmers M I, Jaffray A E and White F 1971 Movements of ammonia following intraruminal administration of urea
or casein. Proc. Nutr. Soc. 30: 7-17.
Dehority B A 1993 Laboratory manual for
classification and morphology of rumen ciliate protozoa. CRC Press, Boca Raton,
FL.
Ffoulkes D and
Preston T R 1978 Cassava or sweet potato forage as combined
sources of protein and roughage in molasses based diets: effect of
supplementation with soybean meal. Trop. Anim. Prod. 3(3): 186-192.
Ffoulkes D, Done F and Preston T R 1978 Cassava forage as a cattle feed: apparent digestibility and
consumption of the whole forage. Trop. Anim. Prod. 3(3): 234-236.
Ffoulkes D and
Preston T R 1979 Digestibility of cassava forage. Trop. Anim.
Prod. 4(1):
110.
Jouany J P and Senaud J 1979 Role of rumen
protozoa in the digestion of food cellulosic materials. Ann. Rec. Vet. 10:
261-263.
Joux F and
LeBaron P 1997 Ecological Implications of an Improved Direct
Viable Count Method for Aquatic Bacteria. Appl. Environ. Microbiol. 63(9):
3643-3647.
Garcia R L, Mejias
R, and Herrera J 1994 Preliminary
results of the combination of cassava and sugar cane foliar area as a
supplement for dairy cows under grazing conditions. Cuban J. Agric. Sci. 28:
41-43.
Garcia R L and Herrera J 1998
Milk production from pastures and
cassava (Manihote sculenta) or sweet potato (Ipomea batata) integral forage
plant supplementation. Cuban J. Agric. Sci. 1998. 32: 29-31.
Kanjanapruthipong J and Leng R A 1998 The effects of dietary urea on microbial populations in the rumen
of sheep. Asian-Aus. J. Anim. Sci. 11(6): 661-672.
Leng R A 1995 Trees-their Role in Animal Nutrition in Developing Countries in the Humid Tropics. University of New England, Armidale, N S W 2351. Australia.
Mom
Seng, Preston T
R, Leng R A and Meulen U ter 2001 Response of young cattle fed
rice straw to supplementation with cassava foliage and a single drench of
cooking oil. Livestock Research for Rural
Development, 9(2): http://www.cipav.org.co/lrrd/lrrd13/4/seng134.htm.
Mooney P and O’Donovan D J 1970 The permeability of the rumen to simple nirogenous compounds.
Biochem. J. 119: 18-19.
Navas C A, Laredo M A, Cuesta A, Anzola H and Leon J C 1993 Evaluation of Enterolopium ciclocarpum as dietary
alternative to eliminate protozoa from the rumen. Livest. Res. Rural Dev. 4(1):
55-63.
Orskov E R Hovel, F D De and Mould F 1980 The use of nylon bag technique for the
evaluation of feedstuffs. Trop. Anim. Prod. 5: 195-213.
Owen E. N and
Jayasuriya M C 1989 Use of crop residues as animal feeds in developing countries. Res.
Dev. Agric. 3: 129-138.
Preston T R and Leng R A 1987 Matching
Ruminant Production Systems with Available Resources in the Tropics and
Subtropics. Penambull Books, Armidale, NSW, Australia.
Queiroz A C, Barbosa M A, Resende F D, Pereria
J C and Dura A R 1998a Supplementation
of corn stover in the feeding of cattle. 1. Intake, dry matter passage rate,
and in situ dry matter and neutral detergent fiber degradability. Bras. Res.
Zootec. 27(2): 381-389.
Queiroz A C, Barbosa M A, Resende F D, Pereria
J C and Dura A R 1998b Supplementation of corn stover in the feeding
of cattle. 2. Ruminal ammonia concentration and ruminal pH. Bras. Res. Zootec.
27(2): 390-396.
Reed J D, McDowell R E van Soest P J and Horvath P J 1982 Condensed Tannins: A factor limiting the use
of cassava forage. J. Sci. Food Agric. 33:
213-220.
Sundstol F and Coxworth E M 1984 Ammonia treatment of straw and other fibrous
by-products as feed. In: Straw and other Fibrous By-products as Animal Feed.
Sundstol F And Owen E (Ed.). Elsevier Scientific Pub. Co.
Amsterdam. Pp. 196-247”.
van Soest P J 1982 Nutritional Ecology
of the Ruminant. 2th Ed Corvalis, O & B Books 374p
van Soest P J, Robertson J B and Lewis B A 1991 Methods for dietary
fiber, neutral detergent fiber and non-starch polysaccharides analyses in
relation to animal nutrition. J. Dairy Sci. 74: 3583-3597.
Wanapat M 1984 Improving rice straw
quality as ruminant feed by urea-treatment in Thailand. In Proceeding of the
International Workshop on Relevance of Crop Residues as Animal Feeds in
Developing Countries. Wanapat, M and
Devendra C (Ed.). Khon Kaen University, Thailand, Nov. 29 – Dec. 2, 1984. Pp.
147 –175”.
Wanapat M 1995 The use of local feed
resources for livestock production in Thailand. In Proceeding of the
International Conference on Increasing Animal Production with Local Resources.
Guo Tingshuang (Ed ). China Forestry Publishing House, Ministry of Agriculture,
China.
Wanapat M., Pimpa O , Petlum A and Boontao U 1997 Cassava hay: A new strategic
feed for ruminants during the dry season. Livestock Research for Rural
Development, 9(2): http://www.cipav.org.co/lrrd/lrrd9/2/metha92.htm.