 |
|
|
| Citation |
Two on-farm experiments were conducted in a suburb of Long Xuyen City, An Giang province, Vietnam, to investigate the effect of diets of rice polishings with taro (Colocacia esculenta) foliage on the growth performance of common ducks. In experiment 1, 168 crossbred common ducks (crosses of an exotic and indigenous breed) were used in a completely randomized design (CRD) with 7 dietary treatments and 3 replicates. The ducks were offered chopped fresh taro leaves ad libitum, supplemented with five levels (3, 4, 5, 6, 7% of live weight [LW] as dry matter [DM]) of a basal diet (soybean meal, rice bran and broken rice) with a premix added There were two other treatments, the basal diet without premix fed at 3% of LW, with free-access to taro leaves, and the basal diet with premix, fed at the equivalent of 7% of LW, with no taro leaves provided. Each experimental unit included 8 ducks, balanced for sex. In experiment 2, in total 80 common ducks were used, with ten treatments, two replicates and four ducks (balanced for sex) per replicate. The dietary treatments were arranged as a 5*2 completely random factorial design, with ingredient ratio (5) and feeding system (2) as factors. The basal diet was high protein rice bran supplemented by five levels of taro silage (20, 30, 40, 50, and 60%), fed to the ducks in mixed or separate form.
In experiment 1, the total dry matter intake was highest on the treatment in which the ducks were fed 7% of LW of the basal diet supplemented by fresh taro leaves ad libitum (110g/day), and lowest in the treatment with 3% of LW of the basal diet and taro leaves ad libitum (84.4g/day). The average daily gain among treatments was significantly different (P<0.05), and was poorest in the treatment 3% of basal diet-taro leaves ad libitum. It was concluded that fresh taro leaves can meet the duck’s requirements of vitamins and minerals. In experiment 2, silage made from taro foliage (leaves and stems) was shown to replace up to 60% of the rice bran in diets for growing ducks without any reduction in growth performance and with positive effects on carcass quality (the weight of abdominal fat decreased as the taro silage intake increased).
For smallholder farmers in the Mekong delta there can be significant economic benefits from the opportunity to fatten common ducks using resources (rice bran and taro foliage) that are widely available in the region and of lower cost than commercial feeds.
Poultry production is a common activity in Southeast Asia, and is a major source of livelihood for over a million people in the rural areas. In the last two decades, Asian duck production has become more important, making up 87% of the world's duck population, and duck meat and egg production has increased more than four times (Chein Tai and Jui-Jane Liu Tair 2001). This expansion has mainly come from the preservation of local breeds and strains, such as the local Muscovy duck and several Vietnamese breeds such as the Co and Tau duck (Duong Thanh Liem 2001), and imports of exotic and improved breeds. Improved ducks have become widely accepted and have increased in number, and are generally called “common ducks”.
Duck production is one component of integrated farming systems which are regarded as being part of a sustainable development in agriculture. Ducks (Anas platyrhynchos) can be integrated with rice, orchards, cash crops, livestock, and fish. Thus, the stakeholders not only can develop their livelihoods without accumulating debts, but also can get extra income through off-farm and non-farm activities (Le Thanh Phong et al 2007). The Mekong Delta, located in the South of Vietnam, is considered as the country’s granary, accounting for 48% of the national rice production (followed by the Red River Delta). Besides, Mekong Delta has a warm ambient temperature and high annual rainfall that is suitable for duck production. Natural resources, including paddy rice fields, canal networks, and plant and grasses, for instance, are advantageous for ducks to increase in number. Ducks can effectively utilize low quality feed (agricultural residues, by-products and insects) and can produce highly nutritional foods for humans (Bui Xuan Men et al 1998). Duck production is diversified into several raising systems according to economic criteria, for example, industrial integrated, medium to large commercial, medium to small commercial and mixed farming systems (integration of rice-ducks, ducks-fish or rice-fish-ducks) or spatial criteria, such as scavenging, semi-confined and confined systems (Edan et al 2006). The large scale system has developed only recently in some areas of the delta. It is generally agreed that better breeds, together with improvements in management of stock health and using local feed resources, as well as other appropriate technologies should enhance sustainable small-scale duck production.
However, the free-raising of ducks in the rice fields or canals (scavenging system) without strict management of outbreaks of diseases is a risk for community health and also duck production. In order to deal with this important issue and create a sustainable duck production, semi-confined and confined systems are being introduced and widely extended, with the aim of limiting the spread of infectious diseases such as Duck Plague and Avian influenza.
An Giang Province, situated in the Mekong Delta is a well- known area for rice production, with 3,519,343 tonnes produced in 2008 (An Giang Statistical Yearbook 2009). The most common duck production system in this area is free grazing in the rice fields, utilizing the leftover rice grains, insects and snails as part of an integrated pest management system (Teo 2001). According to the An Giang Statistical Yearbook (2009), in 2008, there were 4,296,840 poultry, of which 3,437,129 were ducks, raised in An Giang Province.
Annually, rice mills produce large quantities of grain for export, as well as the by-products (rice husk, rice bran and broken rice). The broken rice is not as valuable as rice grain but it also can be exported or used locally for human consumption. Rice bran is the outer layer of the brown rice kernel (after separating the husk) which is removed while milling brown rice to white. Rice bran is a rich source of nutrients and a pharmacologically active compound and is currently used as livestock feed and for oil production (Tahira et al2007). According to Houston (1972), rice bran often occupies 5-8 percent of paddy rice (whole grain). Commonly, in Vietnam, the rice mills have produced three kinds of rice bran: the initial bran (mixed with rice husk fragments) and two types of bran produced in the polishing process which are very fine and have higher nutritional value than the initial bran. In the Mekong Delta, rice bran is cheaper than rice grain and broken kernel so it is the most widely available feed resource for duck production.
Taro can be commonly found growing wild in the Mekong Delta, particularly on the
banks of ponds and along rivers and canals. Traditionally, some taro species (Colocasia
antiquorum and Colocasia gigantea ), which have a large corm or
palatable stem, can be used for home consumption, while Xanthosoma sp.,
Alocasia sp., Alocasia cucullata and Alocasia
macrorrhiza (giant taro/giant elephant
ear) can be used both as human food and animal feed. Wild taro (Colocasia
esculenta) originates from India and Southeastern Asia. It is a perennial
herb 1.5 m tall, with thick stems, very small corms, and with leaf blades around
60 in length and 50 cm in width. Wild taro is very easy to grow, develops
fastest in wet land and is highly resistant to pests and diseases. The wild taro
leaf has a high nutritional value, with 22.5-26.3% crude protein in the dry
matter (DM) (Malavanh Chittavong et al 2008a and Chhay Ty et al 2007).
However, in common with other species of the Aracae family, an anti-nutritional
substance, calcium oxalate, is found in all parts of the plant, causing
irritation in the throat and mouth epithelium and indirectly affecting the feed
intake. The influence of calcium oxalate can be reduced by ensiling with
molasses (Malavanh Chittavong et al 2008b), or by the addition of the stems
without any further additive (Du Thanh Hang and Preston 2010; Nguyen Tuyet Giang
2008).
Two experiments were conducted in a private farm in Long Xuyen City, An Giang Province, Vietnam. The climate is divided into two seasons: the rainy season (from May to November), and the dry season (from December to April). The annual average temperature is 27ºC. The highest mean daily temperature is 35ºC - 37ºC from April to May and the lowest 20ºC - 21ºC, from December to January. The annual rainfall is 1,400-1,500mm.
Experiment 1 was carried out using 168 crossbred common ducks (crosses of an exotic and indigenous breed) in a completely randomized design (CRD) with 7 dietary treatments and 3 replicates. Each experimental unit involved 8 ducks, balanced in sex. The dietary treatments are presented in the table below:
|
Table 1. Experimental treatments |
|||
|
Treatments |
Feeding level |
Taro leaves |
Premix |
|
W7PTL |
7% of LW |
fed ad libitum |
supplied |
|
W7P |
7% of LW |
- |
supplied |
|
W6PTL |
6% of LW |
fed ad libitum |
supplied |
|
W5PTL |
5% of LW |
fed ad libitum |
supplied |
|
W4PTL |
4% of LW |
fed ad libitum |
supplied |
|
W3PTL |
3% of LW |
fed ad libitum |
supplied |
|
W3TL |
3% of LW |
fed ad libitum |
- |
|
LW: live weight; P: premix and TL: taro leaves |
|||
|
Table 2. Composition of the vitamins and minerals premix supplied |
|
|
|
Per kg |
|
Premix of vitamins and minerals |
|
|
Vitamin A |
2,500,000 IU |
|
Vitamin D3 |
500,000 IU |
|
Vitamin E |
1,500 IU |
|
Niacinamide (Vitamin B3) |
5,000 mg |
|
Calcium Pantothenate |
3,000 mg |
|
Vitamin C |
3,300 mg |
|
Riboflavin (Vitamin B2) |
1,200 mg |
|
Vitamin K3 |
1,000 mg |
|
Thiamine (Vitamin B1) |
1,000 mg |
|
Pyridoxine (Vitamin B6) |
550 mg |
|
Folic acid |
440 mg |
|
Biotin (Vitamin B7) |
33,000mcg |
|
Vitamin B12 |
5,500 mcg |
|
Premix of minerals |
|
|
Fe, Cu, Mn, Zn, I2, Co, organic Se |
121,200 mg |
|
Biotin |
18 mg |
|
Dicalcium phosphate (DCP) |
1,000 mg |
Experiment 2 was conducted with common ducks allocated in a 5x2 factorial experiment with a completely randomized design, with 10 dietary treatments and 2 replicates. There were two factors:
Five ratios of rice bran to taro silage: 80:20, 70:30, 60:40, 50:50, 40:60 (DM basis).
Two feeding systems: rice bran - taro silage mixed together, and rice bran - taro silage fed separately.
There were 4 ducks, with 2 males and 2 females, allocated to each experimental unit (replication). The dietary treatments are shown in Table 3.
|
Table 3: Experimental treatments |
||
|
Rice bran: taro silage (DM basis) |
Feeding system |
|
|
Mixed feeding |
Separate feeding |
|
|
80:20 |
80-20M |
80-20S |
|
70:30 |
70-30M |
70-30S |
|
60:40 |
60-40M |
60-40S |
|
50:50 |
50-50M |
50-50S |
|
40:60 |
40-60M |
40-60S |
One-day-old ducks were carefully selected in breeding farms with known origin of the eggs. Male and female ducklings were brooded separately in different cages. The ducklings were fed commercial feeds from the second day until 4 days before starting the trial. The temperature was maintained at 32-34oC for the first 7 days and then reduced steadily to normal ambient temperatures. The ducklings were trained to become used to the experimental feeds from the seventh day by mixing increasing amounts with the commercial feed. Vaccinations against two dangerous diseases (Duck Plague and Avian Influenza) were done, following the biosecure procedure (FAO 2008).
The first trial took 50 days (started when the ducklings reached 21 days of age and finished when they were 70 days of age). The second experiment started when the ducks were 28 days of age and finished when they reached 70 days of age (42 days in duration).
The experimental ducks were kept in 3m2pens in a simple house constructed of bamboo and wire nets. The floor was overlaid with 20 cm of sand for bedding. Feeders and drinkers were put in each cage (Photo 1). Plastic tanks were arranged for bathing.
 |
|
|
In Experiment 1, the confined common ducks were fed a basal diet and chopped fresh taro leaves. The basal diet included three ingredients: rice bran, broken rice and soybean meal. The diets were balanced to approximately 16% crude protein (CP) content.
Wild taro leaves (Photo 2) were harvested and chopped in fresh form before feeding. Rice polishing (rice bran and broken rice) was bought in a local factory and soybean meal was purchased in Afiex (Agriculture and Foods Import – Export) Angiang Feed Mill. The basal diet was mixed with water and fed separately from the chopped taro leaves. The premix of vitamins and minerals was weighed and supplied according to the instructions written on the label.
In Experiment 2, the ducks were fed to appetite, which was planned to be about 6% of live weight as DM. In the mixed feeding, the diet components were mixed together. With separate feeding, rice bran was restricted to 80, 70, 60, 50 and 40% of the planned DM intake; the taro silage was supplied ad libitum in a different feed bowl. The premix of vitamins and minerals was supplied according to the instructions written on the label.
The basal diet was fine rice bran (very small particles) with a high CP content bought from a local factory in Chau Thanh District. Taro petioles and leaves were harvested from plants growing on roadsides and other unused areas. These materials were chopped into 2-3 cm lengths with a knife, partially sun-dried to reduce the moisture to 75-80%, and packed tightly into plastic bags (50 littes capacity). The bags were covered with plastic sheets and stored at room temperature. After 4 days, the taro silage changed color to dark brown with a palatable smell and was then ready for use (Photo 3).
The ducks were fed three times per day (in the morning, at noon and in the afternoon).
Water was freely available in plastic bowls.
 |
 |
|
Photo 2. Taro plant (Colocasia esculenta) |
Photo 3. Taro silage |
Feed supplied was weighed every morning, and the residues collected and weighed the following morning. Representative samples of diets were taken and stored in a refridgerator at 4oC, and the dried samples bulked at weekly intervals and stored for analysis.
Feed offered and refusals were prepared and analyzed for dry matter, organic matter (OM), and crude protein (CP) following the methods of AOAC (1990). Acid detergent fiber (ADF) and neutral detergent fiber (NDF) were determined according to Van Soest et al 1991. Ether extract (EE) was determined by Soxhlet extraction. Samples of taro silage were analyzed for minerals (Ca and P) following HPLC-MS (High performance liquid chromatography - Mass spectrometry) method, and oxalate was determined by the method of Martz et al (1990).
Regression or ANOVA analysis in Minitab software (Minitab 2000) was applied to the data depending on the nature of the observed responses in growth and feed conversion ratio. Sources of variation were treatments and error.
|
Table 4. Chemical composition of feedstuffs |
|||
|
|
Feed ingredient |
||
|
Basal diet |
Fresh taro leaves |
||
|
With premix |
Without premix |
||
|
DM ,% |
89.4 |
88.6 |
13.5 |
|
As % in DM |
|
|
|
|
Ash |
5.70 |
7.00 |
19.8 |
|
CP |
16.7 |
16.4 |
23.4 |
|
NDF |
|
|
37.8 |
|
ADF |
|
|
7.70 |
|
Ca |
1.85 |
1.56 |
1.31 |
|
P |
1.80 |
1.06 |
0.26 |
|
Calcium oxalate |
|
|
0.76 |
|
Carotene, mg/kg DM |
130 |
160 |
1280 |
The level of CP of the basal diets were consistent with the recommendation of the Nutrient Requirements of Poultry (National Academy of Sciences 1994) for White Pekin ducks from two to seven weeks of age(16% CP on DM basis) (Table 3). The CP content of the fresh taro leaves used in this research (23.4%) was higher than in the study of Malavanh Chittavong et al 2008b (22.5%). Fresh taro leaves were higher in neutral detergent fiber (NDF) but lower in acid detergent fiber (ADF) than in Giant taro leaves (Lylian Rodríguez et al 2006). The calcium oxalate content found in the fresh taro leaves was lower than that found in studies carried out by Malavanh Chittavong et al (2008b) and Du Thanh Hang and Preston (2010). The amount of carotene found in fresh taro leaves was higher than that in duckweed (Bui Xuan Men et al 1995)
Daily feed intake of the common ducks increased with the rise in the level of basal feed fed (as % of LW as DM). However, the proportion of CP consumed varied slightly due to the high CP content in fresh taro leaves (24.4% vs 16.7% in the basal diet, DM basis). There was a positive trend in the ADG, which increased with a decrease in fresh taro leaves intake, but the FCR did not significantly differ among treatments (Table 5).
|
Table 5. Daily feed intake and performance traits of ducks fed restricted levels (% of LW as DM) of a basal diet (WP) and fresh taro leaves ad libitum |
||||||
|
|
W3PTL |
W4PTL |
W5PTL |
W6PTL |
W7PTL |
SE/P |
|
Intake, g/day |
|
|
|
|
|
|
|
Basal feed |
31.0a |
45.6b |
60.5c |
73.1d |
83.6e |
1.39/0.001 |
|
Taro leaves |
53.5a |
49.6a |
41.7b |
33.9c |
26.4d |
0.94/0.001 |
|
Total DM |
84.4a |
95.2b |
102b |
107c |
110d |
2.00/0.001 |
|
DM FCR, kg feed/ kg gain |
4.15 |
3.96 |
3.91 |
3.79 |
3.78 |
0.09/0.100 |
|
ADG, g |
20.4a |
24.1b |
26.1c |
28.2d |
29.1d |
0.38/0.0001 |
|
As % in diet DM |
|
|
|
|
|
|
|
Taro leaves |
63.3a |
52.1b |
40.8c |
31.7d |
24.0e |
0.72/0.001 |
|
CP |
22.6a |
21.8b |
20.9c |
20.4d |
19.4e |
0.08/0.001 |
|
abcde Mean values within rows without common superscript are different at P<0.05 W3PTL: ducks were fed equivalent 3% LW with basal feed mixed with premix, fresh taro leaves ad libitum W4PTL: ducks were fed equivalent 4% LW with basal feed mixed with premix, fresh taro leaves ad libitum W5PTL: ducks were fed equivalent 5% LW with basal feed mixed with premix, fresh taro leaves ad libitum W6PTL: ducks were fed equivalent 6% LW with basal feed mixed with premix, fresh taro leaves ad libitum W7PTL: ducks were fed equivalent 7% LW with basal feed mixed with premix, fresh taro leaves ad libitum
|
||||||
It was very clear that there was no difference in the total DM intake as well as FCR and ADG between ducks fed the basal diet equivalent to 3% of LW with added premix (vitamins and minerals) and without premix and with taro ad libitum (Table 6). With equal feed level offered (7% of LW as DM), ducks also consumed more taro leaves, but the FCR was poorer than when the ducks were fed the basal diet only. The result is similar to the report of Bui Xuan Men et al (1995) in which the FCR became poorer when the duckweed offered was increased. This can be explained by the high fibre content in fresh taro leaves causing low digestibility.
|
Table 6. Effects on intake and performance of taro leaves compared with a minerals-vitamins supplement with a basal diet at 3% of LW |
||||||
|
|
W3PTL |
W3TL |
SE/P |
W7P |
W7PTL |
SE/P |
|
Intake, g/day |
|
|
|
|
|
|
|
Basal feed |
31.0 |
30.3 |
0.46/0.388 |
84.3 |
83.6 |
1.66/0.781 |
|
Taro leaves |
53.5 |
53.2 |
1.09/0.839 |
0.0 |
26.4 |
0.51/0.000 |
|
Total DM |
84.4 |
83.5 |
1.48/0.678 |
84.3 |
110 |
1.94/0.001 |
|
DM FCR, kg/kg |
4.15 |
4.23 |
0.20/0.770 |
3.01 |
3.78 |
0.07/0.001 |
|
ADG, g |
20.4 |
19.8 |
0.70/0.598 |
28.0 |
29.1 |
0.31/0.063 |
|
As % of diet DM |
|
|
|
|
|
|
|
Taro leaves |
63.4 |
63.7 |
0.57/0.815 |
0 |
24.0 |
0.41/0.000 |
|
CP |
22.6 |
22.8 |
0.08/0.124 |
18.4 |
19.4 |
0.09/0.001 |
|
W3PTL: ducks were fed equivalent 3% LW with basal feed mixed with premix, fresh taro leaves ad libitum W3TL: ducks were fed equivalent 3% LW with basal feed without premix, fresh taro leaves ad libitum W7P: ducks were fed equivalent 7% LW with basal feed mixed with premix W7PTL: ducks were fed equivalent 7% LW with basal feed mixed with premix, fresh taro leaves ad libitum |
||||||
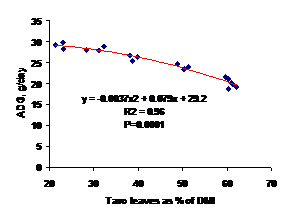
As shown in Figure 2, the proportion of fresh taro silage consumed of total DM intake (DMI) had a negative relationship with ADG (R2=0.96).
|
|
Figure 1. Relationship between proportion of fresh taro leaves and average daily weight gain |
There were no differences in the carcass traits between the
groups with and without premix supplied and the same group fed fresh taro leaves
ad libitum vs basal diet alone (Table 8). However, the feeding level (as
% of LW) had an influence on the slaughter weight, carcass percentage and the
caecum length (Table 7).
|
Table 7. Performance traits of ducks fed restricted levels (% of LW as DM) of a basal diet (WP) and with fresh taro leaves supplied ad libitum |
||||||
|
|
W3PTL |
W4PTL |
W5PTL |
W6PTL |
W7PTL |
SE/P |
|
Slaughter weight, g |
1627a |
1850ab |
1958ab |
2025b |
2052b |
80.8/0.024 |
|
Carcass weight, g |
1095a |
1227ab |
1323ab |
1372b |
1422ab |
53.2/0.010 |
|
Carcass, % |
67.4 |
66.3 |
67.5 |
67.8 |
69.4 |
1.41/0.667 |
|
Chest muscle#, g |
182 |
187 |
186 |
189 |
186 |
4.25/0.839 |
|
Thigh muscle#, g |
67.8 |
69.0 |
69.3 |
70.9 |
84.3 |
4.13/0.109 |
|
Heart#, g |
15.0 |
15.3 |
15.0 |
15.6 |
14.3 |
0.57/0.483 |
|
Liver#, g |
53.4 |
56.2 |
57.0 |
54.0 |
55.3 |
4.31/0.957 |
|
Gizzard#, g |
65.7 |
59.3 |
59.4 |
58.6 |
57.0 |
4.53/0.868 |
|
Small intestine#, cm |
178 |
187 |
191 |
201 |
200 |
9.70/0.748 |
|
Large intestine#, cm |
13.1 |
11.9 |
13.2 |
11.9 |
11.3 |
1.01/0.603 |
|
Caecum#, cm |
28.4a |
21.3b |
18.8b |
18.3b |
16.9b |
1.32/0.014 |
|
Abdominal fat#, g |
5.8 |
11.4 |
16.8 |
17.0 |
25.3 |
3.03/0.085 |
|
# Corrected for carcass weight by covariance ab Mean values within rows without common superscript are different at P<0.05 W3PTL: ducks were fed equivalent 3% LW with basal feed mixed with premix, fresh taro leaves ad libitum W4PTL: ducks were fed equivalent 4% LW with basal feed mixed with premix, fresh taro leaves ad libitum W5PTL: ducks were fed equivalent 5% LW with basal feed mixed with premix, fresh taro leaves ad libitum W6PTL: ducks were fed equivalent 6% LW with basal feed mixed with premix, fresh taro leaves ad libitum W7PTL: ducks were fed equivalent 7% LW with basal feed mixed with premix, fresh taro leaves ad libitum
|
||||||
|
Table 8. Effects of taro leaves on carcass parameters compared with a minerals-vitamins supplement with a basal diet at 3% of LW (W3TL vs W3PTL) and at 7% of LW (W7PTL vs W7P) |
||||||
|
0 |
W3PTL |
W3TL |
SE/P |
W7P |
W7PTL |
SE/P |
|
Slaughter weight, g |
1627 |
1550 |
99.6/0.615 |
2133 |
2052 |
72.6/0.471 |
|
Carcass weight, g |
1095 |
1040 |
69.2/0.606 |
1397 |
1422 |
33.8/0.628 |
|
Carcass, % |
67 |
67 |
2.12/0.933 |
65.5 |
69.4 |
1.26/0.099 |
|
Chest muscle, g |
154 |
151 |
11.4/0.846 |
208 |
205 |
6.87/0.749 |
|
Thigh muscle, g |
55.0 |
51.3 |
6.00/0.688 |
76.7 |
93.2 |
7.48/0.194 |
|
Heart, g |
14.0 |
12.8 |
1.42/0.592 |
15.0 |
15.0 |
0.02/1.000 |
|
Liver, g |
55.0 |
55.0 |
4.08/1.000 |
56.7 |
54.2 |
3.17/0.607 |
|
Gizzard, g |
62.5 |
59.2 |
2.76/0.442 |
55.0 |
59.2 |
2.12/0.238 |
|
Small intestine, cm |
178 |
171 |
9.08/0.631 |
194 |
200 |
7.48/0.572 |
|
Large intestine, cm |
12.9 |
14.3 |
0.53/0.123 |
11.8 |
11.4 |
0.35/0.449 |
|
Caecum, cm |
26.7 |
22.6 |
1.08/0.055 |
17.1 |
18.1 |
0.69/0.364 |
|
Abdominal fat, g |
9.67 |
8.67 |
0.67/0.349 |
20.0 |
24.7 |
2.80/0.305 |
|
W3PTL: ducks were fed equivalent 3% LW with basal feed mixed with premix, fresh taro leaves ad libitum W3TL: ducks were fed equivalent 3% LW with basal feed without premix, fresh taro leaves ad libitum W7P: ducks were fed equivalent 7% LW with basal feed mixed with premix W7PTL: ducks were fed equivalent 7% LW with basal feed mixed with premix, fresh taro leaves ad libitum |
||||||
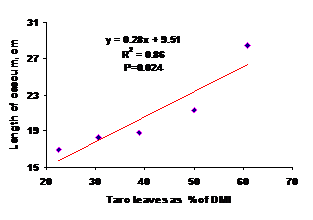
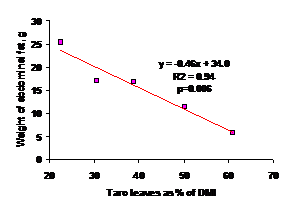
The length of caecum increased as the proportion of fresh taro leaves went up (Figure 2). The fresh taro leaves had a negative impact on the abdominal fat weight; the more taro leaves consumed, the less fat stored up (Figure 3). The results show that the increase in caecum length in response to increase in dietary fiber is linear. The caecum (2 ceaca in pair) in birds is fingerlike and mainly contributes to fibre digestion (but the effect is rather small). Inside the caeca there is a site for the breakdown and fermentation of cellulose that depends on microbial action (Clench and Mathias 1995). The fibrous bulk in the digestive tract could be the reason for the increase of caecum length.
|
|
|
Figure 2. Effect of proportion of fresh taro leaves on the caecum length |
Figure 3. Negative relationship between the proportion of fresh taro leaves and the abdominal fat weight |
The economic analysis shown in Table 9 demonstrates that it
is possible to feed confined common ducks with basal feed (concentrate without a
vitamins- minerals premix) equivalent to 3% of LW and with fresh taro leaves
provided ad libitum with the feed cost per kg weight gain decreasing in
proportion to the amount of taro leaves consumed.
|
Table 9. Estimates of feed costs (VND/kg weight gain) assuming situations of purchase or farm-based production of taro leaves (in VND; about 17,800VND=1USD) |
|||||||
|
|
W3TL |
W3PTL |
W4PTL |
W5PTL |
W6PTL |
W7PTL |
W7P |
|
Experimental conditions (*) |
18,250 |
18,547 |
18,211 |
18,505 |
18,316 |
18,635 |
15,652 |
|
Taro leaves harvested by farmer(**) |
7,979 |
8,438 |
10,275 |
12,416 |
13,677 |
15,233 |
15,652 |
|
(*)1kg basal diet with premix=5200 VND; 1kg basal diet without premix=4800 VND; 1kg fresh taro leaves=600VND= 4,000 VND 90% air-dry basis) (**)Farmers harvest and ensile taro by themselves: 1 kg taro silage=0 VND W3TL: ducks were fed equivalent 3% LW with basal feed without premix, fresh taro leaves ad libitum W3PTL: ducks were fed equivalent 3% LW with basal feed mixed with premix, fresh taro leaves ad libitum W4PTL: ducks were fed equivalent 4% LW with basal feed mixed with premix, fresh taro leaves ad libitum W5PTL: ducks were fed equivalent 5% LW with basal feed mixed with premix, fresh taro leaves ad libitum W6PTL: ducks were fed equivalent 6% LW with basal feed mixed with premix, fresh taro leaves ad libitum W7PTL: ducks were fed equivalent 7% LW with basal feed mixed with premix, fresh taro leaves ad libitum W7P: ducks were fed equivalent 7% LW with basal feed mixed with premix
|
|||||||
|
Table 10. Chemical composition of feed ingredients |
||
|
|
Diet ingredients |
|
|
Rice bran |
Taro silage |
|
|
DM ,% |
89.3 |
24.7 |
|
As % in DM |
||
|
Ash |
8.90 |
3.60 |
|
CP |
13.2 |
18.7 |
|
EE |
16.6 |
|
|
NDF |
40.9 |
30.9 |
|
ADF |
6.60 |
6.11 |
|
Ca |
|
0.15 |
|
P |
|
0.12 |
|
Calcium oxalate |
|
0.31 |
According to the values found for rice bran and taro silage (Table 9), all the experimental diets would supply enough protein to satisfy the demand for growing ducks (16% in DM, Siregar et al 1982). The rice bran had a higher nutritional value than that Chhay Ty et al (2009) reported. The taro silage made from stems and leaves had a lower CP content than the ensiled product made from leaves only (Chhay Ty et al 2007 and Pheng Buntha et al 2008) but the value was similar to that reported by Nguyen Tuyet Giang (2008).
Ensiling can be the best solution, not only for feed preservation but also for reducing calcium oxalate. The calcium oxalate content was reduced 2.45 times in the silage product compared to the concentration in fresh taro leaf (Table 4). This data is in agreement with the reports of Pheng Buntha et al (2008) and Du Thanh Hang and Preston (2010), who reported that ensiling taro leaves was more effective in reducing the calcium oxalate in fresh leaves than other processing methods.
Compared to the planned proportions of rice bran in the feed, the actual amounts consumed were lower in the mixed feeding system and higher in the “separate system” (Table 11).
|
Table 11. Percentage of rice bran in the diets: planned and consumed on the mixed and separate feed system (rest of diet was taro silage) |
|||||
|
Planned |
40 |
50 |
60 |
70 |
80 |
|
Mixed system |
42.4 |
51.3 |
60.2 |
70.2 |
77.1 |
|
Separate system |
34.6 |
40.9 |
57.7 |
62.5 |
67.9 |
The total DM intake and the proportion of the diet as taro silage were higher when the two feeds were mixed together (Table 12). This was probably because of the improved palatability when the rice bran was well mixed with taro silage. This result is different from that reported by Nguyen Thi Kim Dong (2005) when common ducks were fed a concentrate and brewer’s grains mixed or separately. Under a free-choice feeding (feeds are supplied separately), the protein-rich component is acknowledged to be the target of the bird's selection (Pousga et al 2005). This may explain why the taro silage intake was higher when the ducks had free access to the taro silage in the “separate” feeding system. The attractive smell of the taro silage may also have been favored by the ducks. Another explanation is that on the “separate” feeding system there were no residues from the rice bran, whereas in the mixed system there was a rice bran residue equivalent to 5% of the offer level. This implies that if the feeding level had been set higher than 6% of live weight, the ducks on the “separate” system might have eaten more rice bran and less taro silage.
|
Table 12. Daily intake of DM, CP and dietary ingredients of ducks fed taro silage (TS) mixed with a basal diet of rice bran (RB) or offered separately |
|||
|
|
Feeding system |
||
|
Mixed |
Separate |
SE/P |
|
|
Feed intake, g/day |
|
|
|
|
DM RB |
70.6 |
57.7 |
3.89/0.030 |
|
DM TS |
48.8 |
52.1 |
5.70/0.696 |
|
Total DM |
119 |
110 |
2.87/0.027 |
|
Total CP |
18.3 |
17.1 |
0.56/0.138 |
|
As % in diet DM |
|
|
|
|
Rice bran |
60.0 |
52.6 |
4.18/0.227 |
|
Taro silage |
40.0 |
47.4 |
4.18/0.227 |
|
CP |
15.4 |
15.6 |
0.14/0.212 |
As expected, the CP content of the diet decreased slightly
as the proportion of rice bran was increased at the expense of the taro silage
(Tables 13 and 14).
|
Table 13. Effect of ratio of rice bran (RB) and taro silage (TS) on the intake of dietary ingredients and feed constituents (DM and CP) of common ducks under a mixed feeding system |
||||||
|
|
Planned ratio of rice bran: taro silage |
|||||
|
40:60 |
50:50 |
60:40 |
70:30 |
80:20 |
SE/P |
|
|
Feed intake, g/day |
|
|
|
|
|
|
|
DM RB |
56.5a |
64.0b |
74.3c |
81.8d |
76.5e |
0.81/0.0001 |
|
DM TS |
76.7a |
60.8b |
49.2c |
34.8d |
22.8e |
0.79/0.0001 |
|
Total DM |
133a |
125ab |
124b |
117b |
99.2c |
1.56/0.0001 |
|
Total CP |
21.1 |
19.5 |
18.9 |
17.4 |
14.7 |
0.25/0.127 |
|
As % in diet DM |
|
|
|
|
|
|
|
Rice bran |
42.4a |
51.3b |
60.2c |
70.2d |
77.1e |
0.18/0.0001 |
|
Taro silage |
57.6a |
48.7b |
39.8c |
29.8d |
23.0d |
0.18/0.0001 |
|
CP |
16.0a |
15.7b |
15.4c |
15.0d |
14.8e |
0.007/0.0001 |
|
abcde Mean values within rows without a common superscript are different at P<0.05 |
||||||
|
Table 14. Effect of ratio of rice bran (RB) fed separately from taro silage (TS) on the intake of dietary ingredients and feed constituents (DM and CP) of common ducks |
||||||
|
|
Planned ratio of rice bran: taro silage |
|||||
|
40:60 |
50:50 |
60:40 |
70:30 |
80:20 |
SE/P |
|
|
Feed intake, g/day |
|
|
|
|
|
|
|
DM RB |
38.9a |
45.9b |
59.7c |
67.0d |
76.7e |
0.24/0.0001 |
|
DM TS |
73.5a |
66.4b |
43.9c |
40.2d |
36.3e |
1.38/0.0001 |
|
Total DM |
112a |
112a |
104b |
107ab |
113a |
1.31/0.013 |
|
Total CP |
18.2 |
17.9 |
16.0 |
16.4 |
17.0 |
0.24/0.1110 |
|
As % in diet DM |
|
|
|
|
|
|
|
Rice bran |
34.6a |
40.9b |
57.7c |
62.5d |
67.9e |
0.75/0.0001 |
|
Taro silage |
65.4a |
59.1b |
42.3c |
37.5d |
32.1e |
0.75/0.0001 |
|
CP |
16.2 |
16.0 |
15.5 |
15.3 |
15.1 |
0.03/0.0001 |
|
abcde Mean values within rows without a common superscript are different at P<0.05 |
||||||
The average daily gain (ADG) and feed conversion ratio (FCR) were improved when the two feed ingredients were mixed together compared with giving them separately (Table 15). This may have been due to the higher CP content of the diets in the mixed system and the expected higher energy content of the mixed diets, which had higher proportions of rice bran compared with the taro silage.
|
Table 15. Effect of feeding system on the growth performance of common ducks |
|||
|
|
Feeding system |
||
|
Mixed |
Separate |
SE/P |
|
|
Initial weight |
1085 |
1090 |
13.5/0.797 |
|
Final weight |
2208 |
2170 |
13.9/0.078 |
|
ADG, g |
26.3 |
24.5 |
0.32/0.002 |
|
FCR, kg/kg |
4.56 |
4.48 |
0.14/0.661 |
Despite the very wide range in the proportions of taro silage and rice bran there was little variation in the growth rates and feed conversion ratios (Tables 16 and 17) and no relationship between the proportion of rice bran and performance criteria (R2 =0.09 for ADG and 0.30 for FCR).
|
Table 16. Effect of ratio of rice bran:taro silage on live weight change and feed conversion of common ducks under the mixed feeding system |
||||||
|
|
Planned ratio of rice bran: taro silage |
|||||
|
40:60 |
50:50 |
60:40 |
70:30 |
80:20 |
SE/P |
|
|
Initial weight, g |
1113 |
1075 |
1075 |
1063 |
1100 |
28.5/0.727 |
|
Final weight, g |
2238 |
2163 |
2225 |
2175 |
2238 |
29.0/0.320 |
|
ADG, g |
25.7 |
26.6 |
25.0 |
26.9 |
27.2 |
0.74/0.329 |
|
FCR, kg/kg |
5.18a |
4.69ab |
4.95ab |
4.35bc |
3.65c |
0.13/0.002 |
|
abc Mean values within rows without a common superscript are different at P<0.05 |
||||||
|
Table 17. Live weight change and feed conversion ratio of common ducks given rice bran separately from taro silage |
||||||
|
|
Planned ratio of rice bran: taro silage |
|||||
|
40:60 |
50:50 |
60:40 |
70:30 |
80:20 |
SE/P |
|
|
Initial weight, g |
1100 |
1013 |
1138 |
1088 |
1113 |
29.6/0.168 |
|
Final weight, g |
2163 |
2088 |
2225 |
2213 |
2163 |
27.4/0.083 |
|
ADG, g |
25.1 |
24.3 |
24.6 |
23.9 |
24.7 |
0.58/0.686 |
|
FCR, kg/kg |
4.48 |
4.63 |
4.22 |
4.49 |
4.58 |
0.11/0.211 |
There were no differences in live weight at slaughter, carcass percentage and the weight of abdominal fat between the ducks on the mixed and separate feeding systems (Table 18).
|
Table 18. Effect of ingredient ratio (rice bran: taro silage) in two feeding systems on carcass traits and the weight of abdominal fat of common ducks |
|||
|
|
Feeding system |
||
|
Mixed |
Separate |
SE/P |
|
|
Live weight, g |
2158 |
2146 |
16.6/0.615 |
|
Carcass weight, g |
1453 |
1444 |
14.7/0.671 |
|
Carcass, % |
67.3 |
67.3 |
0.29/0.962 |
|
Abdominal fat, g |
15.4 |
14.4 |
1.05/0.523 |
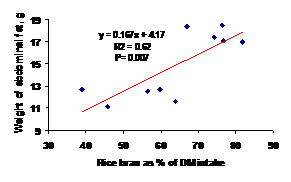
In contrast, there were positive linear relationships between the proportion of rice bran in the diet and the weight of abdominal fat (Figure 4).
|
Table 19. Effect of ingredient ratio (rice bran: taro silage) of confined common ducks under a mixed feeding system on carcass parameters |
||||||
|
|
Planned ratio of rice bran: taro silage |
|||||
|
40:60 |
50:50 |
60:40 |
70:30 |
80:20 |
SE/P |
|
|
Live weight, g |
2125 |
2088 |
2200 |
2163 |
2213 |
26.8/0.089 |
|
Carcass weight, g |
1405abc |
1395b |
1480abc |
1470abc |
1515c |
20.0/0.032 |
|
Carcass, % |
66.1a |
66.8ab |
67.3ab |
68.0ab |
68.5b |
0.40/0.042 |
|
Abdominal fat, g |
12.5 |
11.6 |
17.4 |
17.0 |
18.5 |
1.48/0.067 |
|
abc Mean values within rows without a common superscript are different at P<0.05 |
||||||
|
Table 20. Mean value of received Effect of ingredient ratio (rice bran: taro silage) on the carcass traits of ducks in a separate feeding system |
||||||
|
|
Planned ratio of rice bran: taro silage |
|||||
|
40:60 |
50:50 |
60:40 |
70:30 |
80:20 |
SE/P |
|
|
Live weight, g |
2175a |
2210a |
2090b |
2140a |
2113b |
14.7/0.012 |
|
Carcass weight, g |
1445 |
1493 |
1410 |
1458 |
1415 |
22.2/0.193 |
|
Carcass, % |
66.4 |
67.6 |
67.5 |
68.1 |
67.0 |
0.60/0.439 |
|
Abdominal fat, g |
12.7 |
11.1 |
12.7 |
18.4 |
17.1 |
1.41/0.054 |
|
ab Mean values within rows without a common superscript are different at P<0.05 |
||||||
The increase in abdominal fat would appear to be the consequence of the increase in metabolizable energy content of the diets. A similar result was reported in the study by Nguyen Thi Kim Dong (2005) in which the abdominal fat of growing crossbred ducks increased as the fat content of the diet increased.
|
|
Figure 4. Effect of the proportion of rice bran in the diet on the weight of abdominal fat |
The market price of rice bran (3,600 VND/kg) is around half the price of commercial duck feeds (7,800VND/kg). Taro collected by local farmers was valued at VND 500/kg and an extra VND 500 was added for the cost of ensiling. On a 90% air-dry basis this is equivalent to VND 3,644/kg – the same price as the rice bran but half that of the commercial feed. On this basis the feed cost per kg live weight gain is the same irrespective of the proportion of taro silage (Table 21). However, if collection and ensiling of the taro were to be done by household labour (considered as zero cost), then there would be an economic advantage from using taro foliage to replace rice bran.
|
Table 21. Economic analysis of the effect of level of rice bran supplemented by taro silage for growing common ducks offered mixed or separately (in VND; about 17,800VND=1USD) |
||||||||||
|
Feed cost/kg gain |
Mixed system |
Separate system |
||||||||
|
40:60 |
50:50 |
60:40 |
70:30 |
80:20 |
40:60 |
50:50 |
60:40 |
70:30 |
80:20 |
|
|
Experimental conditions (*) |
18,779 |
16984 |
17,907 |
15,717 |
13,190 |
16,257 |
16788 |
15,271 |
16,238 |
16,553 |
|
Home-made taro silage (**) |
7,907 |
8,661 |
10,728 |
10,993 |
10,131 |
5,580 |
6,817 |
8,766 |
10,103 |
11,195 |
|
(*)1kg rice bran=3600 VND; 1kg taro silage=500 VND (fresh form)+500 VND (ensiling costs)= VND 3,644 on 90% air-dry basis) (**)Farmers harvest and ensile taro by themselves: 1 kg taro silage=0 VND |
||||||||||
There were no health problems on any of the diets and mortality was zero. From the consumer standpoint, the reduction in abdominal fat and the darker yellow colour of the legs and skin, in the ducks fed the higher levels of taro silage, would be considered as an advantage.
The DM and CP intakes were significantly different among treatments (P<0.05) in which ducks were offered the basal diet with five feeding levels and fresh taro leaves were offered ad libitum. The increasing fresh taro leaves intake was to compensate for the reduced amount of basal diet. The result is consistent with the study carried out by Nguyen Thi Kim Dong (2005). The minerals and vitamins content of the fresh taro leaves can meet the requirement of ducks. This could explain why the growth performance of the confined common ducks (FCR and ADG) was similar between the two diets (with and without a premix of vitamins and minerals).
In spite of the fact that ducks can consume high amounts of bulky and fibre-rich feeds, the growth rate was poorer than when ducks received a lower fibre and a higher energy diet. The growth performance of the ducks in this study research was lower than when ducks were fed "A" molasses substituted for broken rice and rice polishings at 15 or 30% in the diet (Bui Xuan Men and Vuong Van Su 1990).
Increasing the proportion of rice bran in the diet, at the expense of the taro silage, should have led to an increase in the concentration of metabolizable energy in the diets, and therefore to improved performance in terms of growth rate and feed conversion. In fact, there were no differences in growth performance, but there were major effects on the carcass, with linear decreases in carcass percentage and in abdominal fat as the level of taro silage in the diet was increased. These responses can be considered as positive in terms of carcass quality of the product and the opportunity to use locally available forage which grows wild in the Mekong Delta.
It is apparent that the ensiled taro foliage (leaf plus stem) has a relatively high nutritive value. The observations in this experiment do not permit conclusions to be made as to the relative nutritional values of the leaf and stem in the ensiled product. Rodríguez and Preston (2009) concluded that ensiling the combined leaf and stem of New Cocoyam (Xanthosoma sagittifolium) was a simpler process than ensiling only the leaf. When leaves were ensiled alone a source of fermentable carbohydrate (sugar cane juice) had to be added. However, it was found that the stem contained appreciable amounts of soluble sugars and thus there was no need for an additive when the leaves and stems were ensiled together. Dao Thi My Tien et al (2010, unpublished data) also observed that the stems of taro (Colocacia esculenta) contained high levels of soluble sugars (up to 40% in the DM) and that this facilitated the ensiling process of the combined taro leaves and stems.
The other important characteristic of the taro stem is that it has a lower content of structural carbohydrates compared with the leaf (30.9 vs 37.8 % NDF in DM and 6.11 vs 7.70% ADF in stem and leaf, respectively). These differences together with the high sugar content will result in a higher energy value for the stem compared with the leaf.
· Fresh taro leaves can meet the duck’s requirement for vitamins and minerals
· Silage made from the leaves and petioles (stems) of wild taro (Colocacia esculenta) can replace up to 60% of the rice bran in diets for growing ducks without any reduction in growth performance and with positive effects on carcass quality.
· For smallholder farmers in the Mekong delta there are likely to be significant economic benefits from the opportunity to fatten common ducks using resources (rice bran and taro) that are widely available in the region and of lower cost than commercial feeds.
The senior author would like to sincerely thank Sida-SAREC and the MEKARN Program for financial support. I also want to express my gratitude to all the people who helped me carry out this study. I would also like to thank the Animal Husbandry and Veterinary Department, Faculty of Agriculture and Natural Resources, Angiang University, for support.
An Giang Statistical Yearbook 2009: Statistical office of An Giang Province.
AOAC 1990: Official methods of analysis. Association of Official Analytical Chemists, Arlington, Virginia, 15th edition, 1298 pp.
Bui Xuan Men and Vuong Van Su 1990: “A” molasses in diets for growing ducks, from http://www.lrrd.org/lrrd2/3/vietnam2.htm
Bui Xuan Men, B Ogle and T R Preston 1995: Use of duckweed (Lemna spp) as replacement for soya bean meal in a basal diet of broken rice for fattening ducks, from http://www.lrrd.org/lrrd7/3/2.htm
Bui Xuan Men, Ogle B and T R Preston 1998 Studies on Duck Production in the Mekong Delta, Vietnam. http://www.ardaf.org/NR/rdonlyres/DE8C434A-E246-48CA-B67A-5AE1E0A4DBEC/0/19969BuiXuanMen.pdf
Chein Tai and Jui-Jane Liu Tai 2001: Future Prospects of Duck Production in Asia. J. Poult. Sci., Vol. 38: 99-112. (2001), from http://www.jstage.jst.go.jp/article/jpsa/38/1/99/_pdf
Chhay Ty, Khieu Borin and T R Preston 2009: Effect of processing taro foliage on growth of pigs fed two grades of rice bran, from http://www.lrrd.org/lrrd21/11/chha21200.htm
Chhay Ty, Khieu Borin, T R Preston and Mea Sokveasna 2007: Intake, digestibility and N retention by growing pigs fed ensiled or dried Taro (Colocasia esculenta) leaf as the protein supplement in basal diets of rice bran/broken rice or rice bran/cassava root meal. Livestock Research for Rural Development. Volume 19, Article #137, from http://www.cipav.org.co/lrrd/lrrd19/9/chha19137.htm
Clench H M and M R John 1995: The avian cecum: a review, from http://elibrary.unm.edu/sora/Wilson/v107n01/p0093-p0121.pdf
Du Thanh Hang and T R Preston 2010: Effect of processing Taro leaves on oxalate concentrations and using the ensiled leaves as a protein source in pig diets in central Vietnam, from http://www.lrrd.org/lrrd22/4/hang22068.htm
Duong Thanh Liem 2001: Biodiversity Approach in Poultry Breeding in Vietnam , University of Agriculture and Forestry - Ho Chi Minh City.
Edan M, Luthi N Bourgeois, Gautier P and Guerne-Bleich E 2006: Free ranging ducks and risks in Avian Flu disease in Vietnam.
FAO 2008: Biosecurity for Highly pathogenic Avian influenza, from http://www.fao-ectad-nairobi.org/IMG/pdf/Biosecurity_and_HPAI.pdf
Houston D F 1972: Rice bran and polish. p. 272-300.
Le Thanh Phong, Henk M J Udo, Martinus E F van Mensvoort, Roel H. Bosma, Le Quang Tri, Dang Kieu Nhan and Akke J van der Zijpp 2007: Integrated Agriculture-Aquaculture Systems in the Mekong Delta, Vietnam: An Analysis of Recent Trends. Asian Journal of Agriculture and Development, Vol. 4, No. 2, from http://www.searca.org/ajad/archives/v-04/02/ajad_v4_n2_phong.pdf
Malavanh Chittavong, T R Preston and B Ogle 2008a: Effect of replacing soybean meal with a mixture of Taro (Colocasia esculenta (L.)Schott) leaf silage and water spinach on apparent digestibility in Mong Cai gilts at two stages of gestation. Livestock Research for Rural Development. Volume 20, supplement, from http://www.lrrd.org/lrrd20/supplement/mala3.htm
Malavanh Chittavong, T R Preston and B Ogle 2008b: Ensiling leaf of Taro (Colocasia esculenta (L.) Shott) with sugar cane molasses. Livestock Research for Rural Development. Volume 20, supplement, from http://www.lrrd.org/lrrd20/supplement/mala1.htm
Martz F A, Weiss M F and Belyea R L 1990: Determination of oxalate in forage by reverse-phase high pressure liquid chromatography. Journal of Dairy Science 73; p474-479, from http://jds.fass.org/cgi/reprint/73/2/474.pdf
National Academy of Sciences 1994: Nutrient Requirements of Poultry. Ninth Revised Edition, 1994, page 42- 43, from http://www.nap.edu/openbook.php?record_id=2114&page=42
Nguyen Thi Kim Dong 2005: An evaluation of brewery waste as a replacement for concentrates in diets for growing crossbred common ducks. Tropical Animal Health and Production. 36 (7): 715-729.
Nguyen Tuyet Giang 2008: Taro (Colocasia esculenta) silage and water spinach as supplements to rice bran for growing pigs, from http://www.mekarn.org/msc2008-10/miniprojects/minpro/giang.htm
Pheng Buntha, Khieu Borin, T R Preston and B Ogle 2008: Digestibility and nitrogen balance studies in pigs fed diets with ensiled taro (Colocasia esculenta) leaf as replacement for fish meal. Livestock Research for Rural Development. Volume 20, supplement, from http://www.cipav.org.co/lrrd/lrrd20/supplement/bunt2.htm
Pousga S, Boly H and B Ogle 2005: Choice feeding of poultry: a review, from http://www.lrrd.org/lrrd17/4/pous17045.htm
Rodríguez L and T R Preston 2009: A note on ensiling the foliage of New Cocoyam (Xanthosoma sagittifolium). Livestock Research for Rural Development. Volume 21, Article #18, from http://www.lrrd.org/lrrd21/11/rodr21183.htm
Siregar A P, Cumming R B and Farrell D J 1982: The nutrition of meat-type ducks. 1. The effects of dietary protein in isoenergetic diets on biological performance, from http://www.publish.csiro.au/paper/AR9820857.htm
Tahira R, Ata-ur-Rehman and Muhammad Anwar Butt 2007: Characterization of rice bran oil, from http://www.jar.com.pk/pdf/45-3-9.pdf
Teo S S 2001 Evaluation of different duck varieties for the control of the golden apple snail (Pomacea canaliculata) in transplanted and direct seeded rice, from http://pestalert.applesnail.net/conferences/icam07/malaysia.pdf
Van Soest P J, Robertson J B and Lewis B A 1991: Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 74: 3583-3597, from http://jds.fass.org/cgi/reprint/74/10/3583