|
|
|
|
Photo 1. Soil |
Photo 2. Sand |
Photo 3. Bio-char |
| Contents | MEKARN MSc 2008-10; Miniprojects |
The "bio-test" with maize as the indicator plant was used to evaluate bio-char as a soil conditioner. The biochar was produced from a gasifier using sugar cane bagasse as the fuel, and was added at levels of 0, 2, 4 ,. 6 and 8% to two types of soil: loam soil and the soil mixed with equal parts of sand. The soil-biochar mixtures were put in plastic bags (2 liter capacity) and 3 seeds of maize planted in each bag. After the seeds had germinated, one or two plants were removed to leave only one plant in each bag. The height and biomass yield of the maize was determined 30 days after planting. The experiment was conducted at Kampong Cham National school of Agriculture, Cambodia from 04 August to 15 September, 2008.
As a result of the high pH of the biochar, the pH values for the different soil-biochar combinations increased as the level of biochar was increased. Increasing the levels of biochar had no effect on the germination rate of the maize, but resulted in linear decreases in height and biomass yield. It is suggested that the negative effects of the biochar were the result of the high soil pH leading to reduced availability of plant nutrients.
Use of chemical fertilizer has become an important feature of crop production, especially in poor soils. However, the price of chemical fertilizer continues to increase, so using another resource to replace chemical fertilizer is very important for poor rural farmers.
Increasing soil organic matter is one way to increase the availability and utilization of plant nutrients. Soil organic matter consists of a variety of components. These include, in varying proportions and many intermediate stages, raw plant residues and microorganisms (1 to 10 percent), "active" organic fraction (10 to 40 percent) and resistant or stable organic matter (40 to 60 percent) also referred to as humus. Organic matter in soil serves several functions. From a practical agricultural standpoint, it is important for two main reasons: first as a "revolving nutrient bank account"; and second, as an agent to improve soil structure, maintain tilth, and minimize erosion ( Lickacz and Penny 2001).
Bio-char is the product of biomass pyrolysis that has been shown to act as a medium for sequestration of carbon when applied to soils (Lehmann et al 2006). It has also been shown that biochar can improve soil quality dramatically (Lehmann et al 2003). Higher nutrient availability for plants is the result of both the direct nutrient addition by the bio-char and greater nutrient retention (Lehmann et al 2003). Bio-char additions not only affect microbial populations and activity in soil, but also plant–microbe interactions through their effects on nutrient availability and modification of habitat (Giller 2001).
Biochar is a high-carbon, fine-grained residue which can be produced either by smoldering biomass utilizing centuries-old techniques (i.e., covering burning biomass with soil and letting it smolder) or through modern pyrolysis processes. Pyrolysis is the direct thermal decomposition of biomass in the absence of oxygen to obtain an array of solid (biochar), liquid (bio-oil) and gas (syngas) products. The specific yield from the pyrolysis is dependent on process conditions, and can be optimized to produce either energy or biochar (Gaunt et al 2008).
However, there is little information on the most
appropriate level of bio-char to add to the soil and of the effects when added
to soils of different characteristics. It is proposed to study these issues
using the "biotest" method. The measurement of the fertility of soils
is usually done by chemical analysis for plant nutrients such as nitrogen (N),
potassium (K), phosphorus (P) and trace elements, as well as physical
measurements of soil structure. Such analyses require access to a laboratory and
this is not feasible for most farmers, especially those with limited
resources. The "biotest" method, which is based on planting some indicator plants in the soil and measuring their
growth and production is one way to measure fertility of soils in an indirect
way (Nguyen Phuc Tien et al 2003). The use of maize (Zea mays) as the
indicator plant in the bio-test has been demonstrated by several researchers (Promkot 2001;
Boonchan Chantaprasarn and Preston 2004).
Growth of maize will be function of the concentration of added biochar and the type of soil in which it is grown..
To measure the effect of
different levels of
bio-char in two types of soil using maize as indicator plant.
The experiment was designed as a randomized complete block (RCB) with a 2*5 factorial arrangement to compare::
There were two blocks (replicates) as follows:
|
Table 1. The treatments |
|||||||||
|
Levels of bio-char in 100% soil |
Levels of bio-char in 50% soil |
||||||||
|
0% |
2% |
4% |
6% |
8% |
0% |
2% |
4% |
6% |
8% |
|
S-0 |
S-2 |
S-4 |
S-6 |
S-8 |
SS-0 |
SS-2 |
SS-4 |
SS-6 |
SS-8 |
|
Table 2. Experiment layout |
||||||||||
|
Block 1 |
S-0 |
SS-2 |
S-6 |
SS-0 |
S-4 |
S-2 |
SS-8 |
SS-6 |
S-8 |
SS-4 |
|
Block 2 |
S-8 |
S-6 |
SS-2 |
S-4 |
S-0 |
SS-6 |
SS-0 |
S-2 |
SS-4 |
SS-8 |
The soil was collected in the campus of Kampong Cham National School of Agriculture (Photo 1). The sand was available from a construction site in the School (Photo 2). The biochar (Photo 3) was produced in Colombia, from a gasifier using sugar cane bagasse as the fuel
|
|
|
|
Photo 1. Soil |
Photo 2. Sand |
Photo 3. Bio-char |
The soil and soil-sand mixtures were put into plastic bags (two liters capacity) which had many holes in the lower part so any excess water could drain out. Three seeds of maize were planted in each bag according to the experimental layout in Table 2. Water was applied uniformly to all bags every morning and evening except on rainy days. When the seeds had germinated one or two plants were removed to leave only one seedling in each bag.
The soil and sand were analyzed for dry matter (DM), organic matter (OM), N and pH; the bio-char was analyzed only for DM and OM at the beginning. The height of the maize plants was measured every 5 days over a total period of 30 days. After 30 days, the plants and roots were removed from the bags (Photo 4), washed free of soil, and the green parts (leaves and stems) and the roots weighed separately 30 minutes later. These components were then chopped and representative samples analyzed for DM, ash and N.
|
|
|
Photo 4. Maize with different levels bio-char added in soil |
Ash and N were determined following standard procedures (AOAC 1990). DM was determined by micro-wave radiation (Undersander et al 1993).
The data were analyzed by Analysis of variance
(ANOVA) using the General Linear Model (GLM) option of the Minitab software
(version 13.3) (Minitab 2003). The sources of
variables in the model were: Soil type, levels of bio-char, interaction soil
type*bio-char level and error. The Tukey test in the Minitab software was used to separate mean values that differed when the F-test
was significant at
P<0.05.
The high pH of the biochar (Table 3) indicates that the ash component was composed mainly of alkaline elements probably potassium, sodium and calcium, as these are the main mineral elements in most plant tissues. As a result, the pH values for the different treatments increased as the level of biochar was increased (Figure 1).
|
Table 3. Chemical composition of soil, sand and bio-char |
|||
|
|
soil |
sand |
bio-char |
|
DM, % |
78.4 |
97.4 |
98 |
| Ash, % in DM | 68.9 | 94.5 | 66.6 |
|
N, % in DM |
0.28 |
|
|
|
pH |
6.5 |
6.0 |
9.5 |
|
Table 4. Amount of carbon and pH in the mixtures of bio-char, soil or sand-soil before and after finishing the experiment |
|||||
|
Bio-char in soil, % |
pH before experiment |
pH after finished experiment |
Weight of carbon, tonnes/ha |
||
|
Soil |
Sand-soil |
Soil |
Sand-soil |
||
|
0 |
6.5 |
6.5 |
5.5 |
6 |
0.0 |
|
2 |
7.5 |
7 |
6 |
6.5 |
8.1 |
|
4 |
8 |
8 |
7 |
7 |
16.2 |
|
6 |
8.5 |
8.5 |
8 |
8 |
24.4 |
|
8 |
8.5 |
9 |
8.5 |
9 |
32.5 |
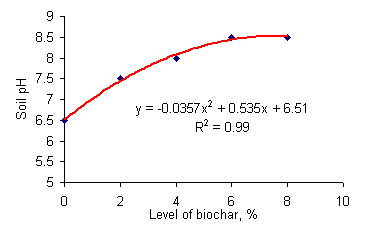 |
| Figure 1. Relationship between level of biochar and pH of the soil |
There were no differences among the treatments in the time for the maize to germinate (Table 4).
|
Table 5. Days to germination of the maize |
||
|
Bio-char levels, % |
Soil |
Soil-sand |
|
0 |
3.5 |
3.5 |
|
2 |
3.65 |
3.35 |
|
4 |
3.7 |
3.15 |
|
6 |
3.85 |
3.75 |
|
8 |
3.5 |
4 |
|
SEM/P |
0.26/0.56 |
|
|
SEM = Standard error of mean, P = Probability level |
||
The height and biomass yield of the maize declined linearly with increasing rates of addition of biochar (Table 5 and Figures 2 and 3). These findings are very different from the results of a similar experiment in Colombia where levels of biochar of 5% led to major increases in maize biomass yield (Figure 4). The major difference between the two experiments was: (i) the the pH of the soil in Colombia was acid (pH 4.0-4.5) compared with a pH of 6.5 in this experiment; and (ii) at 5% addition of biochar the pH was only increased to 6.5-7.0 in the Colombian experiment while in the present experiment, the addition of 4% biochar raised the pH of the soil almost to 8.0. It is likely that the high pH was responsible for the decreased growth rate as it is known that high soil pH reduces availability of phosphorus which is an essential nutrient for plant growth (Brady et al 1996).
|
Table 6. Mean values of the growth of maize in two types of soil with addition of different levels of biochar |
||||
|
Level of bio-char |
Height at 30 days (cm) |
Total biomass (g) |
||
|
Soil |
Soil sand |
Soil |
Soil sand |
|
|
0 |
110 |
104 |
121 |
135 |
|
2 |
84.1 |
103 |
116 |
132 |
|
4 |
95.6 |
53.5 |
108 |
21 |
|
6 |
74.2 |
60.8 |
65.5 |
34 |
|
8 |
42.8 |
52.6 |
65 |
22 |
|
SEM/P |
19.8/0.60 |
44.1/0.455 |
||
|
SEM = Standard error of mean, P = Probability level |
||||
The reduced maize biomass yield in Colombia (Figure 5) in an experiment where levels of biochar exceeded 25%, confirm the negative effects of too high levels of biochar due to the associated increases in soil pH.
|
|
|
|
Figure 2. Relationship between total height of maize and level of bio-char added to two different soils |
Figure 3. Relationship between total biomass of the maize and level of bio-char added to two different soils |

|
|
| Figure 4. Effect of biochar on maize growth in good and eroded soils with and without addition of effluent (Rodriguez et al 2008) | Figure 5. Effect of high levels of biochar on maize growth (Rodriguez et al 2008) |
An advantage of the biotest is that it can be done by farmers to tes the fertility of their soils. However, farmers rarely have access to weigh scales with sufficient precision to weigh small quantities of immature maize plants. However, the height of the maize is easily measured with a simple rule. The data in Figure 6 show that there is a close relationship between the height of the maize and yield of fresh biomass. Thus simple measurements of the height of the maize is sufficient to detect differences in soil fertility. Similar close relationships were observed by Boonchan Chantaprasarn et al (2004) and Tran Thi Bich Ngoc (2005).
|
|
|
Figure 6. Relationship between plant height and total biomass yield for maize |
It is suggested that the negative response was because the soil was already close to neutral (pH 6.5) and the effect of the biochar was to raise the pH to more than 8.0, at which point nutrients such as phosphorus become less available to the plant.
The mini-project was carried out at Kampong Cham National school of Agriculture, Cambodia. We wish to thank the SIDA- SAREC for funding this research - a part of the MSc course through the regional MEKARN project. We also would like to express our gratitude to Dr. Do Van Xe, Mr. Chhay Ty of CelAgrid (Cambodia) and Mrs. Latsamy, and Kampong Cham National school of Agriculture staff and students who provided and prepared the materials for conducting the project.
AOAC 1990 Official methods of analysis. Association of Official Analytical Chemists, Arlington, Virginia, 15th edition, 1298 pp.
Gaunt J L and Lehmann J 2008 Energy Balance and Emissions Associated with Bio-char Sequestration and Pyrolysis Bioenergy Production, 42 ENVTL. SCI. & TECH. 4152, 4155. http://pubs.acs.org/cgi-bin/abstract.cgi/esthag/2008/42/i11/abs/es071361i.html
Giller K E 2001 Nitrogen Fixation in Tropical Cropping Systems, 2nd ed., CAB International, Wallingford.
Lehmann J, Gaunt J and Rondon M 2006 Bio-char sequestration in terrestrial ecosystems – a review, Mitigation and Adaptation Strategies for Global Change (2006), DOI: 10.1007/s11027-005-9006-5. www.css.cornell.edu/faculty/lehmann/publ/MitAdaptStratGlobChange%2011,%20403-427,%20Lehmann,%202006.pdf
Lehmann J, da Silva Jr J P, Steiner C, Nehls T, Zech W and Glaser B 2003 Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments, plant soil, 249, 343-357. www.css.cornell.edu/faculty/lehmann/publ/PlantSoil%20249,%20343-357,%202003%20Lehmann.pdf
Lickacz J and Penny D 2001 Soil Organic Matter. http://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/agdex890
Minitab 2003 Statistical software Version 13.31.
Nguyen Phuc Tien, Ngo Tien Dung, Nguyen Thi Mui, Dinh Van Binh and Preston T R 2003 Improving biomass yield and soil fertility by associations of Flemingia (Flemingia macrophylla) with Mulberry (Morus alba) and cassava (Manihot esculenta) on sloping land in Bavi area. In: Proceedings of Final National Seminar-Workshop on Sustainable Livestock Production on Local Feed Resources (Editors: Reg Preston and Brian Ogle). HUAF-SAREC, Hue City, 25 – 28 March, 2003. Retrieved September 23, 2003, from http://www.mekarn.org/sarec03/tienbavi.htm
Rodriguez Lylian 2008 Effect of biochar on growth of maize in acid soils in Colombia. T R Preston, Personal communication.
Tran Thi Bich Ngoc 2005 Soil bio-test to evaluate comparative fertility of soils and effects of adding earthworm compost. http://www.mekarn.org/MSC2005-07/minpro/ngoc05.htm
Undersander D, Mertens D R and Lewis B A 1993 Forage analysis procedures. National Forage Testing Association. Omaha pp 154.