Cassava (Manihot esculenta, Crantz) leaf meal as
a protein source for young cattle fed rice straw
Ho Thanh Tham
Literature review
Planting cassava in Vietnam
Cassava or tapioca (Manihot esculenta, Crantz), of the family Euphorbiaceae, is an annual root crop that grows widely in tropical and sub-tropical areas. It can easily thrive in sandy-loam soil with low organic matter, receiving low rainfall and high temperatures (Wanapat 1999). In Vietnam, cassava production has steadily increased during recent years and has rapidly changed its role from a food crop to an industrial crop, mainly because of increases in both area planted and yield per hectare. Cassava is the third crop after rice and maize, with about 423.800 ha planted in 2005 (General Statistics Office of Vietnam 2006). Cassava is mostly cultivated in the South-East region of Vietnam (Table 1), which includes Ninh Thuan, Binh Thuan, Binh Phuoc, Tay Ninh, Binh Duong, Dong Nai, Ba Ria-Vung Tau provinces and Ho Chi Minh city. The varieties most popular with farmers are considered to be: Gon, "Japan", "India", KM60, KM94 and KM95 (Phuc et al 1996). The common cassava production system in Vietnam aims at optimising the root yield harvested after a growing period of almost one year, with the foliage being left in the field.
|
Table 1. Planted area of cassava in Vietnam (thousand ha) |
|||
|
Locations |
2003 |
2004 |
2005 |
|
Red River Delta |
7.6 |
7.4 |
7.3 |
|
North East |
47.5 |
49.4 |
49.4 |
|
North West |
37.5 |
40.6 |
42.5 |
|
North Central Coast |
44.5 |
48.4 |
52.8 |
|
South Central Coast |
42.9 |
51.7 |
58.3 |
|
Central Highlands |
65.4 |
70.6 |
88.3 |
|
South East |
109.8 |
114.1 |
118.8 |
|
Mekong River Delta |
10.4 |
6.4 |
6.4 |
|
Whole country |
371.9 |
388.6 |
423.8 |
|
Source: General Statistics Office of Vietnam (2006) |
|||
With increasing demand for high quality feeds for cattle there is a growing interest to also utilize the cassava foliage as a protein supplement, which may lead to the cassava production system developing into a dual-purpose crop (Khang 2004). One hectare of cassava can produce a foliage yield of 0.64 tonnes DM/ha at root harvesting (Khang 2004), which on a national basis would be the equivalent of about 270,000 tonnes cassava foliage DM per year.
Intercropping cassava with a leguminous shrub such as Flemingia (Flemingia macrophylla) improves soil fertility and increases the protein content of the cassava foliage (Dung and Preston 2007). Previously, cassava has been characterized as an "exploitive" crop, destructive of soil fertility. However, when cassava is grown as a component of a farming system, in which livestock and crops are closely integrated, its capacity to "exploit" the nutrients in live stock manure becomes a valuable asset (Preston 2001).
Nutrient composition of cassava leaves
The mineral profile of cassava leaf meal (CLM) prepared from leaves remaining after the harvest of cassava roots showed it to be good sources of most minerals, particularly of calcium and trace minerals. The P and Na contents, however, were low (Ravindran and Ravindran 1988). The chemical composition of fresh cassava leaves, and after ensiling or sun-drying, are presented in Table 2. The CP in the leaves ranged from 23.9 to 34.7% and the fibre from 9 to 14% (both as % of DM) (Phuc et al 1996). While cassava leaf protein is low in sulphur amino acids (Gomez and Valdivieso 1984), the content of most other essential amino acids is higher than in soya bean meal (Eggum 1970). The high protein content and a relatively good profile of essential amino acids are reasons for believing that cassava leaves could be a potential protein source for monogastric animals (Phuc et al 2001), while stems plus petioles or whole plant are more suitable for ruminants (Borin 2005).
|
Table 2. Chemical composition of cassava leaves |
||||||
|
|
% of DM |
References |
||||
|
|
DM |
CP |
ADF |
NDF |
Ash |
|
|
CLM |
89.94 |
20.36 |
20.91 |
27.65 |
7.84 |
Tham et al (2007) |
|
91.72 |
22.54 |
18.85 |
25.6 |
8.53 |
Khang (1999) |
|
|
FCF |
20.12 |
21.51 |
28.8 |
38.17 |
5.78 |
Khang (2004) |
|
29.2 |
18.8 |
34.2 |
51.8 |
6.3 |
Man (2001) |
|
|
20.3 |
16.9 |
37.1 |
48.7 |
6.4 |
Dung (2003) |
|
|
ECF |
39.27 |
20.3 |
37.16 |
50.1 |
6.89 |
Khang (2004) |
|
31 |
21.5 |
34.6 |
51.2 |
5.8 |
Man (2001) |
|
|
CH |
92.4 |
18.9 |
29.7 |
39.5 |
10.7 |
Dung (2003) |
|
93.4 |
24.9 |
27.0 |
34.4 |
6.6 |
Wanapat et al. (1997) |
|
|
PCF |
90.18 |
20.62 |
36.71 |
49.09 |
6.89 |
Khang (2004) |
|
CLMCLM=Cassava leaf mea; FCF=Fresh cassava foliage; ECF=Ensiled cassava foliage ; CH=Cassava hay; PCF=Pelleted cassava foliage |
||||||
Influence of processing methods and storage time on hydrocyanide acid (HCN), and condensed tannins (CT) of cassava leaves
Understanding of the influences on tannin content is essential for the manipulation of tannins to maximise nutritive value for animals (Norton 2000). Cassava leaves have good potential as an animal feed in the tropics on the basis of their protein, amino acid and mineral contents (Ravindran et al 1982). However, CT and cyanide are two anti-nutritional factors in cassava leaves that may reduce the nutritional quality of the leaf meal (Padmaja 1989). Fresh cassava leaves contain high levels of cyanogenic glucosides (Lancaster and Brooks 1983), which after enzymic hydrolysis give rise to toxic hydrocyanic acid. Cyanide content in cassava foliage can be reduced to levels that are safer for animals by drying (Ravindran et al 1987; Phuc et al 2001) or ensiling (Man 2001). Phuc et al (1995) reported that the HCN content (DM basis) was reduced by sun-drying from 190 mg/kg DM in the fresh leaf to 20 mg/kg DM in the leaf meal. Similarly, the HCN content was reduced by drying from 86 mg/kg in the leaf meal dried at 60oC, to 28 mg/kg in the leaf meal dried at 105oC (Phuc et al 2001). At 105oC the drying temperature had a marked effect on the cyanide content of cassava leaves and their consequent toxicity (Ravindran 1993). As has been reported by Wanapat and Rowlinson (2005) that sun-drying eliminated more than 90% of the HCN and enhanced the palatability and long-term storage. Khang (2004) found that cyanide content was reduced by 92% in pelleted cassava foliage and 78% in cassava foliage silage after drying and ensiling, respectively. The effect of three processing methods (drying, chopping and wilting), their combinations and the storage time on HCN and the crude protein (CP) content of CLM were evaluated by Ravindran et al (1987). Fresh cassava leaves contained an average of 1436 mg HCN/kg DM and simple drying (sun- or oven-) eliminated almost 90% of the HCN. A combination of chopping and 3-day wilting before drying proved most effective, lowering the cyanide potential of the final product to about 55 mg/kg DM. The HCN content of CLM declined by 58.2% during an 8-month post-processing storage.
CT was generally found to have a higher value in mature cassava leaves, but levels were lower in cassava hay harvested at a younger stage (Wanapat 2001). Sun-drying of leaves is the method of processing commonly used by farmers.
Tannins in plant leaves are considered to be detrimental when they exceed 4 to 5% in the DM by reducing palatability and digestibility (Kumar and D'Mello 1995). However, at lower levels they can be beneficial by protecting the protein from rumen microbial attack through the formation of insoluble complexes with the protein (Barry 1989). The tannin content of plants is affected by plant species, genotype and stage of growth, and may vary with plant part (leaf, stem, inflorescence, seed), season of growth and other specific environmental factors such as temperature, rainfall, cutting and defoliation by grazing herbivores including insects (Norton 2000). Free tannin contents of cassava leaves were markedly lowered by drying (Padmaja 1989). Similar effects were also found by Khang (2004) and Borin (2005), who reported that sun drying and ensiling reduced tannin contents in cassava foliage, although ensiling after sun wilting was more effective. Tannin content was reduced from 3.51% in fresh cassava foliage to 2.42% and 2.74% after drying and ensiling, respectively. Therefore, wilted and ensiled, or dried and pelleted cassava foliage can be used as a safe protein source in cattle diets to improve the productivity, without negative effects on thyroid gland and liver functions. In their study, Man (2001) demonstrated that a reduction of 25% in the tannin content of fresh cassava tops was found after ensiling; and storage time had little effect on the tannin content.
HCN and tannin contents of cassava leaves processed in different ways are summarized in Table 3.
|
Table 3. HCN and tannins contents of cassava leaves processed in different ways |
||||
|
|
HCN |
Reference |
CT |
Reference |
|
FCF |
840 |
Man (2001) |
40 |
Chantaprasarn (2005) |
|
983 |
Khang (2004) |
35.1 |
Khang (2004) |
|
|
CH |
120 |
Ho Bunyeth (2005) |
31 |
Wanapat et al. (2000) |
|
128 |
Dung (2003) |
23 |
Dung (2003) |
|
|
PCF |
341 |
Khang (2004) |
24.2 |
Khang (2004) |
|
ECF |
408 |
Khang (2004) |
27.4 |
Khang (2004) |
|
292 |
Man (2001) |
45 |
Man (2001) |
|
|
CLM |
- |
|
31 |
Khang (2004) |
|
CT=Condensed tannins; FCF=Fresh cassava foliage; CH=Cassava hay; PCF=Pelleted cassava foliage; ECF=Ensiled cassava foliage ; CLM=Cassava leaf meal |
||||
In the rainy season, it is difficult to sun-dry. Thus, ensiling is an appropriate method during this period, but is less advantageous because it increases labour costs and the risks of unfavourable microbial processes during the time of ensiling and storing, which affect the palatability and nutrient content and may lead to the development of toxic substances (Man 2001). From a practical and economic point of view, sun-drying would be the method of choice in the developing countries of the tropics (Phuc et al 2000).
Use of cassava leaves as a protein supplementation for ruminants
A considerable amount of new research information about the use of cassava as animal feed is becoming available from ongoing research in Vietnam, Thailand and Cambodia. Recent experimental findings on the use of cassava foliage as a protein supplement for pigs (the ensiled leaves), goats and cattle (the fresh or hay foliage), and buffaloes are encouraging and lay the basis for future research and development activities that promise to have a major impact in tropical farming systems. The most immediate prospects for the use of cassava leaf products are in the following areas: (i) low level inclusion of leaf meal in feed formulations for monogastric animals, and (ii) fresh foliage or cassava hay as a protein supplement to low-quality roughages in ruminant feeding.
In Thailand, cassava leaves have been used as a protein source for ruminants, especially for dairy cattle when collected at tuber harvesting time (Wanapat et al 2000). These authors considered that cassava hay was an excellent source of supplemental by-pass protein for dairy cattle especially during the long dry season and had the potential to increase productivity and profitability. The ruminal protein degradation of cassava hay was relatively low (48.8%) which Wanapat et al (2000) ascribed to the presence of tannins forming protein complexes, with the net result that it would act as a source of by-pass protein for digestion in the small intestine. According to Hong et al (2003), supplementation with cassava hay at 2-3 kg/hd/d or its provision as a sole source of roughage in dairy cattle could remarkably reduce concentrate supplementation and increase the fat and protein content of milk thus resulting in increased economic returns. These authors also suggested that cassava hay supplementation in dairy cattle could significantly increase milk thiocyanate which would possibly enhance milk quality and milk storage especially in small holder-dairy farming.
In contrast with the cassava research programme in Thailand, which is mainly based on production and utilization of cassava hay for dry season feeding (Wanapat 2001), in Cambodia the emphasis is on use of the fresh foliage on a year-round basis. For cattle, the emphasis has been on the use of the cassava foliage to supplement untreated rice straw as a fattening system for local yellow cattle. The results were encouraging, especially when the cassava foliage was combined with a single drench of vegetable oil at the beginning of the fattening period (Seng Mom et al 2001).
For cattle, fresh cassava foliage has been successfully fed as the only protein supplement in diets based on molasses-urea (Ffoulkes and Preston 1978). In an experiment with urea-treated rice straw fed to Sindhi x yellow cattle plus 0.72 kg DM/day of Napier grass and 0.26 kg DM/day of cassava root meal per 100 kg LW, provision of 100 g CP/day of fresh cassava foliage per 100 kg LW increased LW gain with an indication of reduced levels of nematode eggs in the faeces (Khuong and Khang 2005).
In an experiment with different levels of wilted cassava foliage fed to growing goats (Vanthong and Ledin 2006), the highest weight gain was recorded with 40% of wilted cassava foliage in the basal diet of Gamba grass (Andropogon gayanus).
In Paper I, it was shown that using CLM as a protein supplement to rice straw sprayed with a mixture of urea and molasses in cattle, led to an increase of total DM intake per kg LW and growth rates that were 73% higher (214 g/day) for cattle given CLM at 0.5% of body weight compared with the control treatment with no CLM (58 g/day). There was a linear increase in LW gain (R2=0.88) as proportion of CLM CP in total CP of the diet increased. The linear nature of the response curve indicates that higher levels of CLM protein might be needed to maximize LW gain.
Various methods for protecting feed protein from degradation by rumen bacteria
The protein value of feeds for ruminants is based on an estimate of the quantity of dietary protein that reaches the sites of enzymic digestion in the intestine (Preston and Leng 1987). Dietary proteins that escape degradation in the rumen are thus a significant factor in determining the protein value of feeds (Aufrère et al 2001). Manget (1997) reported that protein meals with high level of naturally available rumen-undegradable protein (RUP) or bypass protein should be preferred for incorporation in the diet of lactating and growing animals. However, if such resources are non-available or are expensive, protein meals having high rumen degradability can be carefully subjected to heat or formaldehyde treatment to achieve desired level of rumen bypass capacity (Manget 1997). The processing of protein sources to increase the RUP fraction with heat by roasting, extruding, and expelling, and by treating with formaldehyde and lignosulfonate has therefore received much attention recently (Eastridge 2006).
Heating
Heating is one of the factors which may affect the rumen degradability of protein (Dakowski et al 1996). For highly producing ruminants, heat treatment of protein supplements has been used for increasing the amount of dietary protein escaping rumen degradation, and to increase the amino acid pool entering the small intestine (McKinnon et al 1995). However, heating above the optimal temperature may overprotect the protein to a degree where it is neither fermented in the rumen nor digested in the intestine because of the Maillard reaction (Dakowski et al 1996). Thus, the application of heat must be a balance between beneficial and destructive effects.
In Tham et (2007b), it was shown that heating the various sources of leaves to 140oC for 2 hours remarkably increased fraction B3 that is considered to escape the rumen (Sniffen et al 1992). However, it was also apparent that the proportion of the dietary protein in the B3 fraction was not well correlated with the observed nutritive values of the different leaves based on animal feeding trials. Indeed the water hyacinth leaves, which had the highest proportion of the B3 fraction have been shown to be a poor source of protein for growing goats (Sunday 2002). In contrast, the B2 fraction, which appeared to be best correlated with expected animal performance, was decreased markedly by heat treatment.
Formaldehyde treatment
In vivo studies have demonstrated quite clearly that formaldehyde treatment of protein sources can lead to an increased quantity of dietary amino acids reaching the duodenum undegraded (eg: Nishimuta et al 1974). Formaldehyde reduces protein degradability by forming cross-links between protein chains and has anti-microbial properties that may alter the bacterial population (Nagel and Broderick 1992). However, application of formaldehyde is difficult because of its corrosive nature and cost.
Various methods for estimating forage protein degradation in the rumen
Protein degradation information is important according to NRC (1985). Several methods have been performed to obtain reasonably accurate estimates of rumen-degradable protein (RDP) and rumen-undegradable protein (RUP). These methods include in vivo, in situ, and a variety of in vitro methods (Schwab et al 2003).
In vivo
The in vivo method is considered ideal for protein source evaluation because feeds experience normal digestion processes. With in vivo measures, microbial and endogenous nitrogen needs to be distinguished from dietary nitrogen, which can lead to difficulty in estimation of ruminal protein degradability (Vanzant et al 1996). Most importantly, in vivo measures of ruminal protein degradability are not practical for routine evaluation of feeds due to cost, timeliness, and the need for cannulated animals.
In situ or in sacco
The in situ approach is the most widely used research approach for measuring ruminal protein degradation (NRC 2001) because they utilize the actual ruminal environment (Nocek 1988). The in sacco method of Ørskov and McDonald (1979), with various modifications, is one of the most widely adopted methods for measuring rumen degradable and undegradable protein of feeds. In brief, the in situ procedure involves placing multiple samples of a feed into nylon or Dacron polyester bags with a 40 to 60 μm pore size and then placing the bags into the rumen of ruminally cannulated animals. The bags are removed at varying times of ruminal incubation and washed, and the quantity of undigested crude protein is determined. The NRC (2001) suggests that samples be incubated for 0, 2, 4, 8, 16, 24, and 48 h (72 h for forages). Ruminally undegradable protein is that percentage of the original CP remaining in the bag at the defined endpoint of degradation.
The primary shortcoming of the in situ method is that it is labour intensive and requires the use of cannulated animals, both of which makes it a costly method for obtaining the RDP and RUP values for a feedstuff (Schwab et al 2003). Another shortcoming of this method is the disappearance from the bag of soluble proteins and protein in small particles through the pores (Martin 2001).
In vitro
In vitro methods for estimating protein degradability can be broadly classified under the following headings: solubility in buffers containing proteases, ammonia release from rumen fluid incubated in vitro, solubility in buffer and detergents and degradability predicted using near infrared reflectance spectroscopy (NIRS) (White and Ashes 1999).
Several different in vitro enzymatic methods have been
evaluated to identify a method that performs at least as well as in
the in situ method (Schwab et al 2003). Most of the
cell-free enzymes that have been evaluated have been commercial
proteases rather than proteases extracted from mixed ruminal
microbes. A goal has been to identify a protease or mixture of
proteases that would yield estimates of degradation fractions and
rates that are similar to those generated by mixed ruminal
microorganisms. The two most studied enzymes have been
Streptomyces griseus and ficin. In this case, the rate of
protein degradation is calculated from the rate of accumulation of
amino acid and ammonia, the products of protein degradation. The
usual approach has been to correlate estimates of the "extent" of
ruminal protein degradation obtained with the proteases to in
situ estimates of the "extent" of degradation.
The use of in vitro enzymatic methods to predict rates of
protein degradation in the rumen offers laboratory utility and
analytical precision. However, no single method has emerged as
being universally acceptable for all feedstuffs. Challenges
associated with interfering compounds (e.g., starches and fibre),
and identification of the appropriate enzyme/substrate ratio are
still to be resolved.
In vitro methods using rumen fluid are useful for
evaluating relative protein degradability of high protein feeds
(White and Ashes 1999). There are batch and continuous flow
systems. The continuous flow systems are expensive and not commonly
used for routine laboratory analysis of feeds. Batch methods are
relatively cheap, rapid, and can be applied to a large number of
samples simultaneously. They are based on the measurement of net
ammonia release from a feed sample incubated in isolated rumen
culture. The method partly simulates the rumen environment and so
overcomes one of the criticisms of using isolated commercial
proteases. The main disadvantage is the need to keep fistulated
animals to supply rumen fluid. Moreover, the method is not
appropriate for feeds that contain low protein fractions and high
soluble carbohydrate fractions because more nitrogen can be taken
up by the bacteria than released. This defect can be corrected
using 15N to measure nitrogen recycling by bacteria, or
by using chemical inhibitors of nitrogen uptake (chloramphenicol
with hydrazine sulphate) by microbes (Broderick 1987).
Protein solubility in buffer and detergents (In vitro multi-chemical methods)
The multi-chemical approach for quantifying nitrogen fractions
in feedstuffs is the protein fractionation scheme used in the CNCPS
model (Sniffen et al 1992). The CNCPS partitions crude protein into
5 fractions using 3 solvents and a protein-precipitating agent.
Details of the method are described by Licitra et al (1996) (Figure
1).
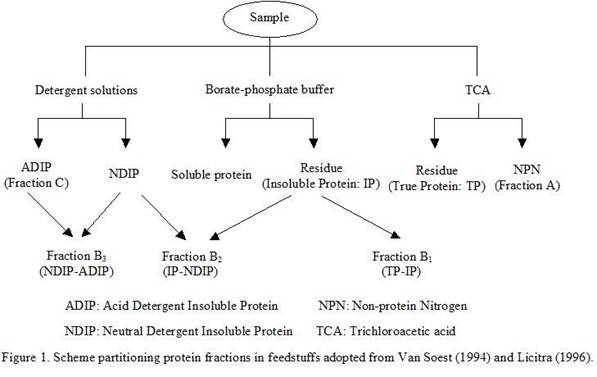
The 5 fractions are fraction A (NPN; soluble in borate-phosphate
buffer but not precipitated with Trichloroacetic acid), fraction B
(true protein). This model divides true protein in feed (using
Trichloroacetic acid precipitate) into three fractions
(B1, B2 and B3) and fraction C
(unavailable true protein or bound protein). The rapidly degradable
fraction (B1) is soluble in phosphate buffer pH 6.8 and
is estimated using the method of Krishnamoorthy et al (1982).
Fraction C is determined by acid-detergent insoluble protein
(ADIP). Protein soluble in neutral detergent solution is described
as degradable (B2) but at an intermediate rate. Protein
insoluble in neutral detergent (NDIP) but soluble in acid detergent
is termed the slowly degradable fraction (B3). Fraction
A is assumed to be 100% degraded in the rumen and fraction C is
assumed to be undegradable.
Shannak et al (2000) utilized the fractionation of feed crude
protein by the CNCPS model as a basis for estimating undegraded
dietary protein (UDP) values of a range of feeds. They claimed that
the values of UDP obtained from in situ trials could be
reliably and accurately predicted from chemical fractionation of
feed crude protein by the CNCPS model. In contrast, the CNCPS model
did not provide realistic predictions of milk production when a
forage (alfalfa silage) was the sole or major dietary ingredient
(Aquino et al 2003).
The use of the CNCPS model to estimate protein fractions of
various feeds used for ruminant production in the Mekong Delta of
Vietnam (Paper II) was not satisfactory as the B3
fraction, considered to best represent the "bypass" protein, was
highest in water hyacinth leaves (which are considered to have a
low nutritive value for ruminants), while insignificant amounts of
the B3 fraction were observed in cassava leaf meal and
in sesbania leaves, which have been shown to significantly increase
growth rates of cattle fed low protein basal diets (eg: CLM in
Paper I, and Ffoulkes and Preston 1978) or to support high rates of
live weight gain in goats fed sesbania leaves as the basal diet
(eg: Dahlanuddin 2001; Nhan 1998)
Characterization of protein sources and beneficial effects of rumen-undegradable protein (RUP) supplements to cattle consuming low quality forage
Broderick (1996) indicated that forages contribute protein to
the cattle in two ways: by providing protein that is degraded by
the ruminal microbes and by providing protein that escapes
microbial breakdown in the rumen (so called rumen bypass protein).
Throughout this paper, the terms "bypass", "escape", or "slowly
degraded" have been used to describe some proteins. These terms
have the same meaning and refer to a protein source's ability to
escape breakdown in the rumen. Excellent source of by-pass protein
for ruminants include blood meal, meat and bone meal, feather meal
(Herold et al 1996), cottonseed meal (Zhang Weixian et al 1994) and
fishmeal (Speedy 2004). According to Stock et al (1996), protein
sources can be divided into four categories: i) high bypass or
slowly degradable protein sources (blood meal, meat meal, fish
meal, corn gluten meal, brewers grains); ii) intermediate bypass
protein sources (cottonseed meal, linseed meal etc.); iii) low
bypass protein sources (soybean meal, corn gluten feed, peanut
meal, sunflower meal, feather meal, rape seed etc.); and iv)
rapidly degradable protein sources (casein, whey, steep liquor
etc.). Research conducted by Promkot and Wanapat (2003) showed that
increasing dietary crude protein levels from 10.5 to 13.7% using
cottonseed meal as the main source to completely replace soybean
meal were beneficial to cows consuming diets based on rice straw
and cassava chips. However, bypass protein meals are usually scarce
and relatively expensive in most countries in the tropics (Leng
2005). Quality protein can be provided sometimes from various crop
residues and by-products of food and drink manufacture, such as
cassava leaves, brewers' grain and maize gluten meal. Speedy (2004)
emphasized that some of these by-products provide a valuable local
source of protein which can be inexpensive, accessible and
continuously available from the local food industry. Greater
utilization of indigenous feed materials is being encouraged for
resource-poor smallholder farmers for increasing ruminant
production. The value of a wide range of tropical feeds was
mentioned, in particular cassava (Manihot esculenta, Crantz)
and the potential of other tree legume sources is being recognized
(Leng 1995). Recent research has shown that hay made from cassava
foliage has a high content of bypass protein presumably because of
its tannin content (Wanapat et al 2000). The potentially very high
yields per hectare (Hong et al 2003; Khang 2004) of cassava foliage
suggest that this may be a practical and valuable source of bypass
protein for ruminant production in South East Asia in the future.
It was recommended that response curves to feeding cassava foliage
to cattle fed local forages and crop residues should be established
and a market created for the sale of foliage meals (Leng 2005). It
is clear that the feed industry and others must continue to look
for alternative, enhanced and cheaper sources of protein for animal
feeds. In addition, the identification of sources of supplemental
protein that will accomplish the goal with maximal nutritional
efficiency while minimizing environmental concerns and economical
costs is important.
In all countries of South East Asia, large ruminants are fed for
a major part of the feed year on low protein, crop residues
(particularly straws), and wasteland grasses. Numerous studies have
shown that on these feeds the priorities are to create an optimum
growth medium in the rumen and to optimize metabolisable protein
(MP) by feeding supplemental by-pass protein (Leng 2005). As
presented in Paper I, it is clear that feed intake was remarkably
increased with the increasing level of CLM in the diet of cattle
consuming rice straw; and that there was a linear positive response
in LW gain to the proportion of the dietary crude protein supplied
as CLM. Similar responses were reported by Sath (2007) who found
that on a basal diet of untreated rice straw plus rumen supplement,
LW gain of Zebu cattle increased with increasing level of protein
from sun-dried cassava foliage. Efficiency of nitrogen utilization
and the cost to benefit ratio for protein supplements may determine
the source and amount of bypass protein to feed to cattle in the
future (Ipharraguerre and Clark 2005).
Conclusions
The review of the literature indicated that:
-
In Vietnam, cassava production has steadily increased during recent years, mainly because of increases in both area planted and yield per hectare. Intercropping cassava with leguminous crops such as Flemingia macrophylla can further increase biomass yield, improve soil fertility and provide food for human consumption, while the residue could be used as supplemental feed, especially during the dry season.
-
Cassava leaves are an excellent multi-nutrient source for animals and have the potential to increase the productivity and profitability of sustainable livestock production systems in the tropics, especially ruminant production. Cassava leaves can provide not only protein, but also appear to have a role as a gastrointestinal anthelmintic agent.
-
Drying and ensiling are effective ways of reducing the toxicity of cassava products, enhancing the palatability and long-term storage, with the reduction being higher after sun-drying.
-
Protein meals having high degradability can be subjected to heat or formaldehyde treatment to achieve desired level of rumen bypass capacity.
-
Several methods have been developed to predict levels of rumen-degradable protein (RDP) and rumen-undegradable protein (RUP), including in vivo, in situ, and a variety of in vitro methods.
-
The CNPNS method which aims to identify and quantify the crude protein fractions that best describe the potential bypass protein does not appear to be appropriate for use with legume forages such as alfalfa silage or tropical plant leaves such as CLM, sesbania and water hyacinth.
References
Aquino D L, Tedeschi L O, Lanzas C, Lee S S and Russell J B
2003 Evaluation of CNCPS predictions of milk production of
dairy cows fed alfalfa silage. Departments of microbiology and
animal science. Cornell University and ARS/USDA, Ithaca, NY
14853.
Aufrère J, Dominique Graviou, Melcion J P and
Demarquilly C 2001 Degradation in the rumen of lupin (Lupinus
albus L.) and pea (Pisum sativum L.) seed proteins-Effect of heat
treatment. Anim. Feed Sci. Technol. 92, 215-236.
Barry T N 1989 Condensed tannins. Their role in ruminant
protein and carbohydrate digestion and possible effects upon the
rumen ecosystem. In the roles of protozoa and fungi in ruminant
digestion, pp. 153-169 (J. V. Nolan, R. A. Leng and D. I. Demeyer,
editors). Armidale, NSW: Penambul Books.
Barry T N, McNeill M D and McNabb W C 2001 Plant
secondary compounds; their impact on nutritive value and upon
animal production. In: Proceedings of the XIX International
Grassland Congress. Brazilian Society of Animal Husbandry,
Piracicaba, pp. 445-452.
Bhat P N and Bansil P C 1999 Grains and roughage
production and its utilization in Asia-Australia region - Review.
Asian-Aus. J. Anim. Sci. 12 (3): 481-492.
Borin, K 2005 Cassava foliage for monogastric animals -
Forage yield, digestion, influence on gut development and nutritive
value. Doctoral thesis, Swedish University of Agricultural
Sciences, Uppsala.
Broderick G A 1987 Determination of protein degradation
rates using a rumen in vitro system containing inhibitors of
microbial nitrogen metabolism. Br. J. Nutr. 58:
463-476.
Broderick G A 1996 Improving utilization of forage
protein by the lactating dairy cow. Informational Conference with
Dairy and Forage Industries. US Dairy Forage Research
Center.
Chantaprasarn B 2005 Study on effects of different
harvest intervals on cassava foliage (cassava hay) and root yield
and effects of sunflower oil supplementation in cassava hay
based-diets for lactating dairy cows. MSc thesis in the programme
"Tropical Livestock Systems". SLU, Dept of Animal nutrition and
management, Uppsala, Sweden.
Chenost M and Sansoucy R 1989 Nutritional characteristics
of tropical feed resources: natural and improved grasslands, crop
residues and agro-industrial by-products. In "Feeding dairy cows in
the tropics". http://www.fao.org/ag/aGa/agap/FRG/AHPP86/Chenost.pdf.
Dahlanuddin 2001 Forages commonly available to goats
under farm conditions on Lombok Island, Indonesia. Livestock
Research for Rural Development (13) 1. http://www.cipav.org.co/lrrd/lrrd13/1/dahl.htm.
Dakowski, P., Weisbjerg, M. R., and Hvelplund, T., 1996.
The effect of temperature during processing of rape seed meal on
amino acid degradation in the rumen and digestion in the intestine.
Anim. Feed Sci. Technol. 58, 213-226.
Dung N T 2003 Evaluation of cassava intercropping systems
and cassava hay as a feed for growing goats. MSc thesis in the
programme "Tropical Livestock Systems". SLU, Dept of Animal
nutrition and management, Uppsala, Sweden.
Dung N T and Preston T R 2007 Effect of increasing area
of cassava (Manihot esculenta Crantz) relative to Flemingia
(Flemingia macrophylla) on biomass yield, soil fertility and
soil erosion. Livestock Research for Rural Development.
19(2), http://www.cipav.org.co/lrrd/lrrd19/2/dzun19022.htm.
Eastridge M L 2006 Major advances in applied dairy cattle
nutrition. J. Dairy Sci. 89: 1311-1323
Eggum B O 1970 The protein quality of cassava leaves. Br.
J. Nutr. 24, 761-768.
Elwakeel E A, Titgemeyer E C, Drouillard J S and Armendariz C
K 2006 Evaluation of ruminal nitrogen availability in liquid
feeds. Anim. Feed Sci. Technol. (2006),
doi:10.1016/j.anifeedsci.2006.10.002.
Ffoulkes D and Preston T R 1978 Cassava or sweet potato
forage as combined sources of protein and roughage in molasses
based diets: effect of supplementation with soybean meal. Trop.
Anim. Prod. 3(3), 186-192.
General Statistics Office 2006 Statistical yearbook of
Vietnam. Statistical Publishing House. Hanoi.
Gomez G and Valdivieso M 1984 Cassava for animal feeding:
Effect of variety and plant age on production of leaves and roots.
Anim. Feed Sci. Technol. 11, 49-55.
Herold D, Klopfenstein T and Klemesrud M 1996Evaluation
of animal byproducts for escape protein supplementation. Nebraska
beef cattle report, University of Nebraska Cooperative
Extension.
Ho Bunyeth 2005 Cassava foliage as supplement for goats
fed paragrass (Brachiaria mutica) in full confinement, or
with grazing in semi-confinement. MSc thesis in the programme
"Tropical Livestock Systems". SLU, Dept of Animal nutrition and
management, Uppsala, Sweden.
Hong N T T, Wanapat M, Wachirapakorn C, Pakdee P and
Rowlinson P 2003 Effects of timing of initial cutting and
subsequent cutting on yields and chemical compositions of cassava
hay and its supplementation on lactating dairy cows. Asian-Aus. J.
Anim. Sci. 16, 1763-1769.
Ipharraguerre I R and Clark J H 2005 Impacts of the
source and amount of crude protein on the intestinal supply of
nitrogen fractions and performance of dairy cows. J. Dairy Sci. 88:
(E. Suppl.): E22-E37.
Khang D N 1999 Effects of cassava leaf meal on rumen
environment and as a replacement for cotton seed meal in napier
grass for dairy cows. MSc thesis in the programme "Tropical
Livestock Systems". SLU, Dept of Animal nutrition and management,
Uppsala, Sweden.
Khang D N 2004 Cassava foliage as a protein source for
cattle in Vietnam. Doctoral thesis Swedish University of
Agricultural Sciences, Uppsala.
Khuong L H and Khang D N 2005 Effect of fresh cassava
foliage on growth and feacal nematoda egg counts in Sindhi x yellow
cattle fed urea-treated rice straw basal diet. Workshop-seminar
"Making better use of local feed resources" (Editors: T. R. Preston
and B. Ogle) MEKARN-CTU, Cantho, 23-25 May, 2005.
Klopfenstein T J, Mass R A, Creighton K W and Patterson H H
2001 Estimating forage protein degradation in the rumen. J.
Anim. Sci. 79, E208-E217.
Krishnamoorthy U C, Muscato T V, Sniffen C J and Van Soest P
J 1982 Nitrogen fractions in selected feedstuffs. J. Dairy Sci.
65:217.
Kumar R and DMello J P F 1995Antinutritional factors in
forage legumes. In: Tropical legumes in animal nutrition. CAB
International.
LancasterP A and Brooks J E 1983 Cassava leaves as
human food. Econ. Bot., 37: 331-348.
Leng R A 1991 Feeding strategies for improving milk
production of dairy animals managed by small-farmers in the
tropics. In "Feeding dairy cows in the tropics". FAO Animal
production and health paper 86.
Leng R A 1995 Evaluation of tropical feed resources for
ruminant livestock. First FAO Electronic Conference on Tropical
Feeds and Feeding Systems. pp. 23-53. Disponible en: http://www.fao.org/ag/aga/agap/frg/ECONF95/PDF/EVALU.PDF.
Leng R A 2005 Metabolisable protein requirements of
ruminants fed roughage based diets. In proceedings of AHAT/BSAT
International Conference: Integrating Livestock-Crop systems to
meet the challenges of globalisation (Editors: P. Rowlinson, C.
Wachirapakorn, P. Pakdee, M. Wanapat), November 14-18, 2005, Khon
Kaen, Thailand.
Licitra G, Hernandez T M and Van Soest P J 1996
Standardization of procedures for nitrogen fractionation of
ruminant feeds. Anim. Feed Sci. Technol. 57,
347-358.
Man N V 2001 Better use of local forages for dairy cattle
in Vietnam: Improving grasses, rice straw and protein rich forages.
Doctoral thesis Swedish University of Agricultural Sciences,
Uppsala.
Manget Ram Garg 1997 Role of bypass protein in feeding
ruminants on crop residue based diet - Review. Asian-Aus. J. Anim.
Sci. 11 (2), 107-116.
Martin M 2001 Degradation of crude protein from the
S-fraction in the rumen fluid. Department of Animal Nutrition and
Management. Swedish University of Agricultural Sciences. Uppsala.
McKinnon J J, Olubobokun J A, Mustafa A, Cohen R D H and
Christensen D A 1995 Infuence of dry heat treatment of canola
meal on site and extent of nutrient disappearance in ruminants.
Anim. Feed Sci. Technol. 56, 243-252.
Nagel S A and Broderick G A 1992 Effect of formic acid or
formaldehyde treatment of alfalfa silage on nutrient utilization by
dairy cows. J. Dairy Sci. 75: 140-154.
National Research Council 1985 Ruminant nitrogen usage.
National Academy Press, Washington, D.C.
National Research Council 2001 Nutrient requirements of
dairy cattle. 7th revised edition. National Academy Press,
Washington, DC.
Nhan N T H 1998
Utilization of some forages as a protein
source for growing goats by smallholder farmers. Livestock
Research for Rural Development 10 (3). http://www.cipav.org.co/lrrd/lrrd10/3/nhan2.htm.
Nishimuta J F, Ely D G and Boling J A 1974Ruminal bypass
of dietary soybean protein treated with heat, formalin and tannic
acid. J. Anim. Sci. 39, 952-957.
Nocek J E 1988 In situ and other methods to estimate
ruminal protein and energy digestibility: A review. J. Dairy Sci.
71: 2051.
Norton B W 2000 The significance of tannins in tropical
animal production. In: Proceedings of international workshop on
"Tannins in livestock and human nutrition" (Ed. J. D. Brooker),
Adelaide, Australia, May 31-June 2, 1999. ACIAR proceeding No. 92,
171pp.
Ørskov E R and McDonald I 1979 The estimation of
protein degradability in the rumen from incubation measurements
weighted according to rate of passage. J. Agric. Sci., 92,
499-503.
Padmaja G 1989 Evaluation of techniques to reduce
assayable tannin and cyanide in cassava leaves. J. Agric. Food
Chem. 37, 712-716.
Phuc B H N, Ogle B and Lindberg J E 2000Effect of
replacing soybean protein with cassava leaf protein in cassava root
meal based diets for growing pigs on digestibility and N retention.
Anim. Feed Sci. Technol. 83, 223-235.
Phuc B H N, Ogle B and Lindberg J E 2001Nutritive value
of cassava leaves for monogastric animals. International workshop
on "Current research and development on use of cassava as animal
feed". (Editors: T. R. Preston, B. Ogle and M. Wanapat) Khon Kaen
University, Thailand, July 23-24, 2001. http://www.mekarn.org/procKK/phuc.htm.
Phuc B H N, Ogle B, Lindberg J E and Preston T R 1996 The
nutritive value of sun-dried and ensiled cassava leaves for growing
pigs. Livestock Research for Rural Development. 8 (3). http://www.cipav.org.co/lrrd/lrrd8/3/phuc83.htm.
Phuc B H N, Lai N V, Preston T R, Ogle B and Lindberg J E
1995 Replacing soya bean meal with cassava leaf meal in cassava
root diets for growing pigs. Livestock Research for Rural
Development. 7 (3). http://www.cipav.org.co/lrrd/index.html.
PrestonT R 2001 Potential of cassava in integrated
farming systems. International workshop on "Current research and
development on use of cassava as animal feed". (Editors: T. R.
Preston, B. Ogle and M. Wanapat) Khon Kaen University, Thailand,
July 23-24, 2001.
http://www.mekarn.org/procKK/pres.htm
PrestonT R and Leng R A 1987 Matching ruminant
production systems with available resources in the tropics and
sub-tropics. Penambul Books Armidale, Queensland 4380,
Australia.
Promkot C and Wanapat M 2003 Ruminal degradation and
intestinal digestion of crude protein of tropical protein resources
using nylon bag technique and three-step in vitro procedure in
dairy cattle. Livestock Research for Rural Development. 15 (11).
http://www.cipav.org.co/lrrd/lrrd15/11/prom1511.htm.
Ravindran V 1993 Cassava leaves as animal feed: Potential
and limitations. J. Sci. Food Agric. 61(2), 141-150.
Ravindran G and Ravindran V 1988 Changes in the
nutritional composition of cassava (Manihot esculenta Crantz)
leaves during maturity. Food Chemistry. 27, 299-309.
Ravindran V, Erwin T Kornegay, Sundara B Rajaguru, David R
Notter 1987 Cassava leaf meal as a replacement for coconut oil
meal in pig diet. J. Sci. Food Agric. 41(1), 45-53.
Ravindran V, Kornegay E T and Rajaguru A S B
1987Influence of processing methods and storage time on the
cyanide potential of cassava leaf meal. Anim. Feed Sci. Technol.
17, 227-234.
Ravindran V, Kornegay E T, Webb K E, Rajaguru A S B 1982
Nutrient characterisation of some feedstuffs from Sri Lankar. J.
Nat. Agric. Soc. Ceylon. 19, 19-32.
Reed J D, McDowell R E, Van Soest P J and Horvath P J
1982 Condensed tannins: A factor limiting the use of cassava
forage. J. Sci. Food Agric. 33, 213-220.
Sath K 2007 Effect of levels of sun-died cassava foliage
on growth performance of cattle fed rice straw. MSc thesis in the
programme "Tropical Livestock Systems". SLU, Dept of Animal
nutrition and management, Uppsala, Sweden.
Schwab C G, Tylutki T P, Ordway R S, Sheaffer C and Stern M D
2003 Characterization of proteins in feeds. J. Dairy Sci. 86:
(E. Suppl.), E88-E103.
Seng Mom, Preston T R, Leng R A and Meulen U ter 2001
Response of young cattle fed rice straw to supplementation with
cassava foliage and a single drench of cooking oil. Livestock
Research for Rural Development 13 (4). http://www.cipav.org.co/lrrd/lrrd13/4/seng134.htm.
Shannak S, Sudekum K H, Susenbeth A 2000 Estimating
ruminal crude protein degradation with in situ and chemical
fractionation procedures. Anim. Feed Sci. Technol. 85:
195-214.
Sniffen C J, O'Connor J D, Van Soest P J, Fox D G and Russell
J B 1992 A net carbohydrate and protein system for evaluating
diets: II. Carbohydrate and protein availability. J. Anim. Sci. 70,
3562-3577.
Speedy A W 2004 Overview of world feed protein needs and
supply. In "Protein sources for the animal feed industry". Expert
consultation and workshop Bangkok, 29 April - 3 May 2002. Food and
Agriculture Organization of The United Nations.
Rome.
Stock R, Mader T and Klopfenstein T 1996New protein
values for ingredients used in growing cattle rations. http://ianrpubs.unl.edu/beef/index.htm.
Sunday A Dada 2002 The utilization of water hyacinth
(Eichhornia crassipes) by West African dwarf (wad) growing
goats. Afr. J. Biomed. Res. (4), 147-149.
Trach N X 1998 The need for improved utilisation of rice
straw as feed for ruminants in Vietnam: An overview. Livestock
Research for Rural Development 10 (2). http://www.cipav.org.co/lrrd/lrrd10/2/trach102.htm.
Vanthong P and Ledin I 2006 Effect of feeding different
levels of wilted cassava foliage (Manihot esculenta, Crantz)
on the performance of growing goats. Small Ruminant Research, doi:
10.1016/j.smallrumres.2006.05.009.
Vanzant E S, Cochran R C, Titgemeyer E C, Stafford S D, Olson
K C, Johnson D E and Jean G St 1996 In vivo and in
situ measurement of forage protein degradation in beef cattle.
J. Anim. Sci. 74, 2773-2784.
Waltz D M and Stern M D 1989 Evaluation of various
methods for protecting soya-bean protein from degradation by rumen
bacteria. Anim. Feed Sci. Technol. 25, 111-122.
Wanapat M 1999 Feeding of ruminants in the tropics based
on local feed resources. Khonkaen Publ. Comp. Ltd., Khonkaen,
Thailand. 236pp.
Wanapat M 2001 Role of cassava hay as animal feed in the
tropics. International workshop on "Current research and
development on use of cassava as animal feed". (Editors: T. R.
Preston, B. Ogle and M. Wanapat) Khon Kaen University, Thailand,
July 23-24, 2001. http://www.forum.org.kh/~mekarn/proc-cass/wana3.htm.
Wanapat M, Pimpa O, Petlum A and Boontao U 1997Cassava
hay: A new strategic feed for ruminants during the dry season.
Livestock Research for Rural Development 9 (2): http://www.cipav.org.co/LRRD/lrrd9/2/metha92.htm.
Wanapat M, Pimpa O, Sripuek W, Puramongkol T, Petlum A,
Boontao U, Wachirapakorn C and Sommart K 2000 Cassava hay: an
important on-farm feed for ruminants. In: Proceedings of
international workshop on "Tannins in livestock and human
nutrition" (Ed. J. D. Brooker), Adelaide, Australia, May 31-June 2,
1999. ACIAR proceeding No. 92, 171pp.
Wanapat M and Rowlinson P 2005 Potential use of cassava
to provide ruminant feeds. In proceedings of AHAT/BSAT
International Conference: Integrating Livestock-Crop systems to
meet the challenges of globalisation (Editors: P. Rowlinson, C.
Wachirapakorn, P. Pakdee, M. Wanapat), November 14-18, 2005, Khon
Kaen, Thailand.
White C L and Ashes J R 1999 A review of methods for
assessing the protein value of grain fed to ruminants Aust. J.
Agric. Res. 50, 855-869.
Zhang Weixian, Gu Chuan Xue, Frands Dolberg and Peter M
Finlayson 1994 Supplementation of ammoniated wheat straw with
hulled cottonseed cake. Livestock Research for Rural Development
(6) 1. http://www.cipav.org.co/lrrd/lrrd6/1/china1.htm.