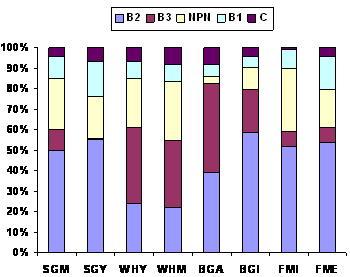Estimates of protein fractions of various heat-treated feeds in
ruminant production
Ho Thanh Tham, Ngo Van Man* and T R Preston**
Department of Animal Husbandry, College of
Agriculture and Applied Biology,
Cantho University, Cantho City, Vietnam
httham@ctu.edu.vn
*Deparment of Animal Nutrition, Nong Lam University,
Ho Chi Minh City, Vietnam
**UTA - TOSOLY - Finca Ecológica, Morario -
Guapota - AA # 48, Socorro, Santander, Santander del Sur,
Colombia
Abstract
Leaves of Cassava (Manihot esculenta Crantz) (CLM),
Sesbania (Sesbania grandiflora) (SG), Leucaena (Leucaena
leucocephala) (LL), Gliricidia (Gliricidia sepium) (GS)
and Water hyacinth (Eichorniacrassipes)
(WH) were subjected to heating at 60 (control), 100 or
140oC for 2 hours. The experimental treatments were laid
out in a 5x3 factorial arrangement with 3 replications. The protein
fractions of the leaves were estimated using The Cornell Net
Carbohydrate and Protein System (CNCPS) model.
Heating the leaves to temperatures of 140ºC for 2 hours
reduced the proportion of the protein in the A (NPN) and
B2 (some fraction B2 escapes to the lower
gut) fractions and increased the B3 (slowly rumen
degradable fraction). The highest value for the B3
fraction was in water hyacinth (44% of the total crude protein)
while in the other leave it was less than 10%. By contrast, the
B2 fraction was low in water hyacinth (less than 20%)
and high in the other leaves and especially in cassava leaf meal
where it accounted for 66% of the total crude protein.
The implication from these findings of are that the
B2 fraction may be a better indicator of the probable
bypass protein value of leaves than the B3
fraction.
Key words: By-pass protein, CNCPS, heat treatment, protein fractions, rumen degradation.
Introduction
Protein requirements for high rates of growth in ruminants
cannot be met solely from microbial protein synthesis in the rumen;
therefore, supplementation with high quality rumen undegradable
protein is necessary (Preston and Leng 1987; McNiven 2002). Due to
the high cost of protein supplements, ways and means of protecting
the protein from degradation in the rumen whilst retaining the high
digestibility is an urgent priority (Leng 1991). Many experiments
have demonstrated the beneficial effects of the technological
processing of feeds, particularly heat treatment, introduced by
Chalmers et al (1954) (cited by Manget 1997), in reducing the
degradation of the crude protein in the rumen without decreasing
digestibility in the small intestine (Aufrère et al 2001). For
highly producing ruminants, heat treatment of protein supplements
has been used for increasing the amount of dietary protein escaping
rumen degradation, and to increase the amino acid pool entering the
small intestine (Faldet et al 1991).
Various approaches are available to assess the ruminal
degradability of protein in feedstuffs, which include in
vivo, in sacco, and in vitro methods (Elwakeel et
al 2006). The in vivo method is considered ideal for protein
source evaluation because feeds experience normal digestion
processes. With in vivo measures, microbial and endogenous N
needs to be distinguished from dietary N, which can lead to
difficulty in estimation of ruminal protein degradability (Vanzant
et al 1996). Most importantly, in vivo measures of ruminal
protein degradability are not practical for routine evaluation of
feeds due to cost, timeliness, and the need for cannulated animals.
The in sacco method is the most widely used method for
estimating ruminal protein degradation because it is less expensive
and simpler than in vivo methods (Ørskov et al 1980).
However, soluble protein and protein in small particles can leave
the bags through the pores without complete degradation (Martin
2001).
The Cornell Net Carbohydrate and Protein System (CNCPS) (Chalupa
et al 1991; Sniffen et al 1992) is one of the schemes developed for
the fractionation of protein in feeds. There is some information
on crude protein content of various feedstuffs collected in the
Mekong Delta (Dung 1996), and on the fractions derived by the CNCPS
system (Dung 2001). However, fractionation of the protein by the
CNCPS system in cassava leaves, and the influence of heat treatment
on these fractions, have not yet been reported.
The characterization of the CP fractions in the CNCPS system in this system is as follows:
Fraction A is non-protein nitrogen (NPN), B is true protein, and C is unavailable true protein or bound protein. Fraction B is further divided into three fractions (B1, B2, and B3) that are believed to have different rates of ruminal degradation. Fractions A and B1 are soluble in borate phosphate buffer and are rapidly degraded in the rumen. Fraction B2 is fermented in the rumen at lower rates than buffer-soluble fractions, and some fraction B2 escapes to the lower gut. Fraction B3 is believed to be more slowly degraded in the rumen than are Fractions B1 and B2 because of its association with the cell wall; a larger proportion of B3 is thus believed to escape the rumen. Fraction C is the ADIP, and is highly resistant to breakdown by microbial and mammalian enzymes, and it is assumed to be unavailable for the animal (see Table 1).
The main purpose of the research described in this paper was to use the CNCPS model to estimate: (i) the protein fractions of cassava leaf meal, and other protein-rich leaves, some of which have been used for ruminants in the Mekong Delta of Vietnam; and (ii) the degree to which these fractions are affected by heat treatment.
|
Table 1. Partition of protein fractions in feedstuffs |
||||
|
Fraction |
Classification (*) |
Abbreviation |
Enzymatic degradation |
Estimation or definition |
|
Non-protein nitrogen |
A |
NPN |
Not applicable |
Not precipitated |
|
True protein |
- |
TP |
- |
Precipitate with TCA |
|
True soluble protein |
B1 |
BSP |
Fast |
Buffer soluble but precipitable (TP-IP) |
|
Insoluble protein |
- |
IP |
- |
Insoluble in buffer |
|
Neutral detergent soluble protein |
B2 |
IP-NDIP |
Variable |
Difference between IP and protein insoluble in neutral detergent (ND) |
|
ND insoluble protein but soluble in acid detergent |
B3 |
NDIP-ADIP |
Variable to slow |
Protein insoluble in neutral detergent but soluble in acid detergent |
|
Insoluble in acid detergent |
C |
ADIP or ADIN |
Indigestive |
Includes heat-damaged protein and nitrogen associated with lignin |
|
* According to Pichard and Van Soest (1977) and Van Soest (1994). |
||||
Materials and methods
Location of the study area
The experiment was conducted in the animal nutritional
laboratory of the Department of Animal Husbandry, College of
Agriculture and Applied biology, Cantho University, Cantho City,
Vietnam. The duration of this study was 3 months, from October 2006
to December 2006.
Treatments and design
The factors in a 5x3 factorial arrangement with three
replications in a randomized complete block were:
Source of leaf materials:
-
Cassava (Manihot esculenta, Crantz) (CLM),
-
Sesbania (Sesbania grandiflora) (SG),
-
Leucaena (Leucaena leucocephala) (LL),
-
Gliricidia (Gliricidia sepium) (GS),
-
Water hyacinth (Eichornia crassipes) (WH).
Heat treatment during 2 hours at:
-
60oC,
-
100oC,
-
140oC.
Samples
Cassava leaf meal was made from cassava leaves, which were
bought in Tayninh province. The leaves were collected from the
field after harvesting the roots and sun-dried for 2 to 4 days. Sun
drying consisted of spreading the leaves on the ground and turning
them over while exposed to the sun, resulting in cassava leaf meal
for direct feeding or storage.
The other leaves were harvested in September 2006 from areas
surrounding Cantho city. These plant protein sources were prepared
in the same way as for cassava leaf meal, by drying under sunlight.
After sun-drying, the leaves were heated in an oven maintained at
temperatures of 60, 100 or 140oC for 2 hours. The heated
samples were then ground to pass a 1 mm screen and stored in a
freezer until analyzed.
Measurements and analysis
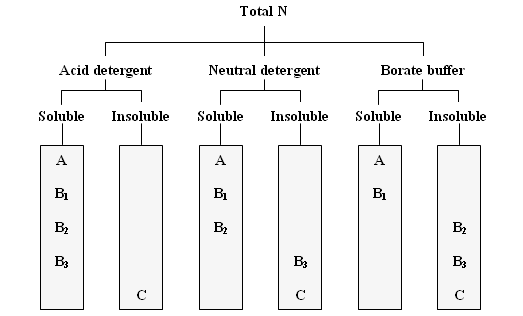
Samples of feed were determined for dry matter (DM) and crude protein (CP) using procedures described by AOAC (1990). The neutral detergent fiber (NDF) and acid detergent fiber (ADF) were determined according to the procedure of Van Soest et al (1991). Crude protein fractionation was performed according to the Cornell Net Carbohydrate and Protein System (Figure 1 and Table 1).
|
|
| A = Soluble in buffer; B1 = soluble in buffer and precipitated by TCA; B3 = insoluble in buffer, insoluble in neutral detergent solution but soluble in acid detergent solution; B2 = insoluble in buffer but soluble in neutral and acid detergent solutions; C = insoluble in buffer and both neutral and acid detergent solutions |
| Figure 1. Analyses of crude protein fractions using borate phosphate buffer and acid detergent and neutral detergent solutions (Roe et al 1990; Sniffen et al 1992). |
To separate the true protein (TP) and non-protein nitrogen (NPN) (Fraction A), trichloroacetic acid (TCA) was used according to the method described by Licitra et al (1996). The TP was separated from the NPN by precipitation with TCA (final concentration 10%). Filtering was done by gravity and NPN was calculated as the difference between total forage N and the N content of the residue after filtration.
Buffer soluble protein was defined as the true protein soluble in a borate-phosphate buffer at pH 6.7-6.8 (Krishnamoorthy et al 1982). Samples were filtered by gravity through Whatman #541 filter papers for subsequent Kjeldahl nitrogen analysis. The insoluble N fraction after filtration was defined as the buffer insoluble protein (IP) fraction. Soluble true protein (Fraction B1) was calculated as the difference between TP and IP.
Neutral detergent soluble protein (Fraction B2) was estimated as the difference between IP and protein insoluble in neutral detergent (NDIP). The amount of soluble fibre-bound CP (Fraction B3) was calculated as CP in NDF minus acid detergent insoluble CP.
Acid detergent fiber (ADF) was prepared according to Van Soest et al (1991) with filtering by gravity on 12.5 cm Whatman # 541 filter papers. Residual N x 6.25 on the filter paper (Acid detergent insoluble protein: ADIP) was classified as Fraction C.
The values of CP in all the fractions including NPN were calculated as g N x 6.25 kg-1 CP.
Statistical analysis
The effect of heat treatment on the protein fractions of samples was analyzed using the General Linear Model (GLM) option of the Minitab software (Minitab release 13.1 2000), as shown below:
Yijk = µ + Ai +
Bj + ABij + eij
where Yijk is the protein fraction. µ the mean
value, Ai the source of the leaf materials,
Bj the effect of heating (60, 100 or 140oC),
ABij the interaction between leaf sources and heating
level, and eij the error.
Results
Effect of heat treatment
Dry matter (DM) and crude protein (CP) content
After heating followed by grinding most of the samples heated at
60ºC tended to absorb more moisture than those heated at 100
and 140ºC (Table 2).
|
Table 2. Effect of heating on residual DM (g/kg feed) in leaf samples |
|||||
|
|
60oC |
100oC |
140oC |
SEM |
P |
|
CLM |
869a |
907b |
907b |
3.6 |
0.001 |
|
SG |
904a |
941b |
962c |
2.7 |
0.001 |
|
LL |
901 |
905 |
914 |
5.2 |
0.261 |
|
GS |
893a |
907ab |
909b |
2.5 |
0.007 |
|
WH |
894a |
910b |
961c |
3.7 |
0.001 |
|
abc Mean within rows with differing superscript letters are significantly different (P<0.05). |
|||||
Heating at high temperature (100 and 140oC) appeared to have very little effect on total crude protein content with slight reductions at 140oC for some of the leaves (Table 3).
|
Table 3. Effect of heating on crude protein in leaf samples (g/kg DM) |
|||||
|
|
60oC |
100oC |
140oC |
SEM |
P |
|
CLM |
255a |
242b |
242b |
1.81 |
0.004 |
|
SG |
318a |
316a |
301b |
2.33 |
0.004 |
|
LL |
302a |
296ab |
289b |
2.01 |
0.012 |
|
GS |
217 |
212 |
210 |
5.23 |
0.695 |
|
WH |
246 |
244 |
240 |
2.41 |
0.355 |
|
abc Mean within rows with differing superscript letters are significantly different (P<0.05). |
|||||
Non-protein nitrogen (NPN - Fraction A)
In the control treatment (60oC) the highest value for NPN was in SG (465 g/kg CP) and the lowest one was in CLM (114 g/kg CP) (Table 4) This component decreased markedly with the gradual increase of heating temperature. In the high temperature treatment (140oC) the reductions were 12, 15, 19, 38 and 58% for SG, LL, WH, CLM and GS, respectively compared with the treatment at 60oC.
|
Table 4. Effect of heating on Fraction A (NPN) in leaf samples (g/kg CP) |
|||||
|
|
60oC |
100oC |
140oC |
SEM |
P |
|
CLM |
114a |
111a |
71b |
4.81 |
0.001 |
|
SG |
465a |
452a |
411b |
8.47 |
0.010 |
|
LL |
390a |
375a |
333b |
5.8 |
0.001 |
|
GS |
186a |
170a |
78b |
8.89 |
0.001 |
|
WH |
142a |
133ab |
115b |
6.01 |
0.050 |
|
abc Mean within rows with differing superscript letters are significantly different (P<0.05) |
|||||
Fraction B1
The fraction B1 was decreased markedly by heating to 140oC (Table 5) with major differences among the leaves, the ranking from high to low being WH>GS>LL>CLM>SG.
|
Table 5. Effect of heating on Fraction B1 (g/kg CP) in leaf samples |
|||||
|
|
60oC |
100oC |
140oC |
SEM |
P |
|
CLM |
42 |
29 |
3 |
20.54 |
0.453 |
|
SG |
19 |
10 |
7 |
13.42 |
0.878 |
|
LL |
126a |
42b |
25b |
5.85 |
0.001 |
|
GS |
138a |
134a |
57b |
17.17 |
0.027 |
|
WH |
155a |
67b |
62b |
10.45 |
0.001 |
|
abc Mean within rows with differing superscript letters are significantly different (P<0.05) |
|||||
Fraction B2
Values for fraction B2 were higher than for B1, and also declined with increase in temperature except in the case of leucaena (Table 6).
|
Table 6. Effect of heating on Fraction B2 (g/kg CP) in leaf samples |
|||||
|
|
60oC |
100oC |
140oC |
SEM |
P |
|
CLM |
663a |
651a |
359b |
16 |
0.001 |
|
SG |
434a |
450a |
309b |
15.76 |
0.001 |
|
LL |
421a |
497b |
545b |
10.96 |
0.001 |
|
GS |
553a |
503a |
244b |
13.98 |
0.001 |
|
WH |
213a |
263b |
15c |
11.28 |
0.001 |
|
abc Mean within rows with differing superscript letters are significantly different (P<0.05) |
|||||
Fraction B3
The B3 fraction, which is considered to be the most important as a potential source of bypass protein, was increased markedly by heat treatment in all the leaves with the exception of leucaena which showed only minor changes due to heating (Table 7).
|
Table 7. Effect of heating on Fraction B3 (g/kg CP) in leaf samples |
|
|||||
|
|
60oC |
100oC |
140oC |
SEM |
P |
|
|
CLM |
93a |
109a |
429b |
24.13 |
0.001 |
|
|
SG |
45a |
54a |
241b |
5.06 |
0.001 |
|
|
LL |
93 |
96 |
100 |
5.19 |
0.669 |
|
|
GS |
22a |
84b |
494c |
9.53 |
0.001 |
|
|
WH |
437a |
485b |
760c |
5.06 |
0.001 |
|
|
abc Mean within rows with differing superscript letters are significantly different (P<0.05) |
||||||
Fraction C
The results of heat treatment on fraction C were variable with increases for CLM and GS but little change in the other leaves (Table 8).
|
Table 8. Effect of heating on fraction C (g/kg CP) in leaf samples |
|||||
|
|
60oC |
100oC |
140oC |
SEM |
P |
|
CLM |
88a |
100a |
138b |
3.18 |
0.001 |
|
SG |
36 |
34 |
32 |
1.25 |
0.134 |
|
LL |
37a |
55b |
60b |
2.04 |
0.001 |
|
GS |
101a |
109ab |
128b |
4.51 |
0.016 |
|
WH |
54 |
53 |
48 |
1.8 |
0.128 |
|
abc Mean within rows with differing superscript letters are significantly different (P<0.05) |
|||||
Comparisons among the leaves
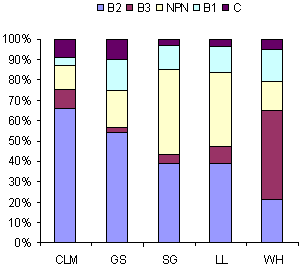
There were major differences among the leaves for the different crude protein fractions (Tables 2 through 8). For the control treatment at 60ºC (Figure 2) the A fraction (NPN) showed high values for sesbania and leucaena and low values for the other leaves. In contrast, the B3 fraction accounted for only a small proportion of the crude protein in all the leaves except for water hyacinth for which it accounted for almost half of the crude protein. For the B2 fraction the highest values were for cassava leaf meal, the lowest were for water hyacinth with intermediate values for gliricidia, sesbania and leucaena.
|
|
|
Fraction C is the insoluble fraction, B1 is the
soluble fraction, B2 is the slowly fermented fraction
and B3 the fraction not fermented in the rumen but
supposedly digestible in the intestine. |
|
Figure 2. Fractionation of the protein in leaves (heated
for 2 hours at 60ºC) according to the CNCPS model.
|
Discussion
Effect of heating
The reduction in the NPN component due to heating presumably was due to the loss of amino acids as observed by Kari et al (2000) for soybean meal and fish meal subjected to heat treatment of 135oC for 30 minutes. Jirí et al (1990) reported that one of the first noticeable changes of proteins on heating (even at temperatures around 100oC) is the loss of labile amino acids such as cystine and lysine. Lysine is one of the most temperature sensitive amino acids; it is often destroyed at levels 5 to15 times higher than the other amino acids (Dakowski et al 1996).
The low values for the B1 fraction agree with the
report of Pichard and Van Soest (1977), that in harvested forages
the B1 fraction of the total protein is low Caballero et
al (2001) indicated that B1 increased with maturity of
forages and decreased from fresh to dry which is in agreement with
the results of the current study.
The increase in the B3 fraction at the expense of
B2 as a result of heating, was probably due to the
denaturation of the proteins as this has been shown to reduce their
solubility (Van Soest 1994). Heat treatment has been shown to
differ in its efficacy with different protein meals. When groundnut
cake was heated at 150oC, protein solubility was reduced
from 23.6 to 10.4 percent. However, in soybean meal, under similar
conditions, the reduction in solubility was only from 14.3 to 10.3
percent (Walli 1995 cited by Manget 1997). The effect of heating on
leaf proteins is likely to be different according to the degree of
cross linkages with the fibre (Van Soest 1994) and the formation of
insoluble complexes with compounds such as tannins (Barry et al
2001).
Effect of source of leaves
In the CNCPS model, fraction B3 is believed to be
more slowly degraded in the rumen than are fractions B1
and B2 and is thus believed to escape the rumen
fermentation (Sniffen et al 1992). However, in our study the
highest value for the B3 fraction was in water hyacinth
(44% of the total crude protein) while in the other it was less
than 10%. By contrast, the B2 fraction was low in water
hyacinth (less than 22%) and high in the other leaves and
especially in cassava leaf meal where it accounted for 66% of the
total crude protein. Similar values for B3 and
B2 in water hyacinth (37% and 24%, respectively) and in
Sesbania grandiflora (10% and 50% for B3
and B2, respectively) were reported by Dung (2001)
(Figure 3). Sesbania grandiflora has been shown to
support growth rates in goats of over 100 g/day (Nhan, 1998) while
in Paper I of this thesis, growth rates in cattle were linearly
increased when cassava leaf meal was used as the supplement to
urea-sprayed rice straw. Using the in vitro
pepsin-pancreatin technique to evaluate rumen undegraded protein of
cassava hay, Promkot and Wanapat (2003) reported that in cows fed
with urea-treated rice straw, the value of rumen undegradable
protein (expressed as % of total CP) was 45.4 and very similar to
that in cottonseed meal which is known to be one of the best
sources of bypass protein for growing cattle (Zhang Weixian et al
1994).
It is relevant to note that the CNCPS model did not provide
realistic predictions of milk production when a forage (alfalfa
silage) was the sole dietary ingredient (Aquino et al
2003).
|
|
|
SGM (Sesbania grandiflora, mature), SGY
(Sesbania grandiflora, young); WHY (Water hyacinth, young), WHM (Water hyacinth, mature); BGA (Brewer's grain, artisanal), BGI (Brewer's grain,
industrial); FMI (Fish meal, industrial), FME (Fish meal,
export). |
|
Figure 3. Fractionation of the protein in leaves and
protein meals according to the CNCPS model (Dung
2001). |
Conclusions
-
The implication from the findings of our study, and those of Dung (2001), are that the B2 fraction may be a better indicator of the probable bypass protein value of leaves than the B3 fraction.
-
Heating the leaves to temperatures of 140ºC for 2 hours reduced the proportion of the protein in the A (NPN) and B2 fractions and increased the B3 fraction. However, the relationship of these changes to the nutritive value of the crude protein in the leaves may well be negative.
Acknowledgements
Financial support from SIDA-SAREC is grateful acknowledged and
the authors would like to thank Ms. Nguyen Thi Ngan and Mr. Nguyen
Thiet for their help in laboratory analyses.
References
AOAC 1990
Official methods of analysis (15th edition),
Washington, DC. Volume 1: 69-90.
Aquino D L, Tedeschi L O, Lanzas C, Lee S S and Russell
J B 2003 Evaluation of CNCPS predictions of milk production of
dairy cows fed alfalfa silage. Departments of microbiology and
animal science. Cornell University and ARS/USDA, Ithaca, NY
14853.
Aufrère J, Dominique Graviou, Melcion J P and
Demarquilly C 2001 Degradation in the rumen of lupin (Lupinus
albus L.) and pea (Pisum sativum L.) seed proteins - Effect of heat
treatment. Anim. Feed Sci. Technol. 92, 215-236.
Barry T N, McNeill M D and McNabb W C 2001 Plant
secondary compounds; their impact on nutritive value and upon
animal production. In: Proceedings of the XIX International
Grassland Congress. Brazilian Society of Animal Husbandry,
Piracicaba, pp. 445-452.
Caballero R, Alzueta C, Ortiz L T, Rodríguez M L, Barro
C and Rebol A 2001 Carbohydrate and protein fractions of fresh
and dried common vetch at three maturity stages. Agron. J., 93:
1006-1013.
ChalupaW, Sniffen C J, Fox D G and Van Soest P J
1991 Model generated protein degradation nutritional
information. Proc. Cornell Nutr. Conf., Dept. Animal Science,
Cornell University, Ithaca, NY. pp. 44-51.
Dakowski P, Weisbjerg M R and Hvelplund T 1996 The
effect of temperature during processing of rape seed meal on amino
acid degradation in the rumen and digestion in the intestine. Anim.
Feed Sci. Technol. 58, 213-226.
Dung N N X 1996 Identification and evaluation of
non-cultivated plants used for livestock feed in the Mekong delta
of Vietnam. MSc. Thesis. SwedishUniversityof Agricultural Sciences. Uppsala.
Dung N N X 2001 Evaluation of green plants and
by-products from Mekong Delta with emphasis on fibre utilisation by
pigs. Doctoral thesis. Swedish University of Agricultural Sciences.
Uppsala.
ElwakeelE A, Titgemeyer E C, Drouillard J S and
Armendariz C K 2006 Evaluation of ruminal nitrogen availability
in liquid feeds. Anim. Feed Sci. Technol. (2006),
doi:10.1016/j.anifeedsci.2006.10.002.
Faldet M A, Voss V L, Broderick G A and Satter L D 1991
Chemical, in vitro and in situ evaluation of heat-treated soybean
proteins. J. Dairy Sci. 74, 2548-2554.
Jirí Davídek, Jan Velísek and Jan
Pokorný 1990 Chemical changes during food processing.
Developments in food science 21. Elsevier.
Kari LjØkjel, Odd Magne Harstad and Anders Skrede
2000 Effect of heat treatment of soybean meal and fish meal on
amino acid digestibility in mink and dairy cows. Anim. Feed Sci.
Technol. 84, 83-95.
Krishnamoorthy U C, Muscato T V, Sniffen C J and Van
Soest P J 1982 Nitrogen fractions in selected feedstuffs. J.
Dairy Science. 65:217.
Leng R A 1991 Feeding strategies for improving milk
production of dairy animals managed by small-farmers in the
tropics. In "Feeding dairy cows in the tropics". FAO Animal
production and health paper 86.
Licitra G, Hernandez T M and Van Soest P J 1996
Standardization of procedures for nitrogen fractionation of
ruminant feeds. Anim. Feed Sci. Technol. 57,
347-358.
Manget Ram Garg 1997 Role of bypass protein in
feeding ruminants on crop residue based diet - Review. Asian-Aus.
J. Anim. Sci. 1998. Vol. 11, No. 2: 107-116.
Martin M 2001 Degradation of crude protein from the
S-fraction in the rumen fluid. Department of Animal Nutrition and
Management. Swedish University of Agricultural Sciences. Uppsala.
McNiven M A, Prestløkken E, Mydland L T and
Mitchell A W 2002 Laboratory procedure to determine protein
digestibility of heat-treated feedstuffs for dairy cattle. Anim.
Feed Sci. Technol. 96, 1-13.
Minitab User's guide 2000 Minitab Release 13.1 for
windows, Windows* 95/98/2000. Minitab Inc., State College
Pennsylvania, USA.
NhanN T H 1998 Utilization of some forages as a
protein source for growing goats by smallholder farmers. Livestock
Research for Rural Development 10 (3).
http://www.cipav.org.co/lrrd/lrrd10/3/nhan2.htm.
Ørskov E R, Hovell F D D and Mould F L 1980
The use of the nylon bag technique for the evaluation of
feedstuffs. Tropical Anim. Prod. 5, 195-213.
Pichard G and Van Soest P J 1977 Protein solubility
of ruminant feeds. Proc. Cornell Nutr. Conf. Department of Animal
Science, Cornell University, Ithaca, NY, pp. 91-98.
Preston T R and Leng R A 1987Matching ruminant
production systems with available resources in the tropics and
sub-tropics. Penambul Books Armidale, Queensland 4380,
Australia.
PromkotC and Wanapat M 2003 Ruminal degradation
and intestinal digestion of crude protein of tropical protein
resources using nylon bag technique and three-step in vitro
procedure in dairy cattle. Livestock Research for Rural Development
(15) 11. http://www.cipav.org.co/lrrd/lrrd15/11/prom1511.htm.
Roe M B, Sniffen C J and Chase L E 1990 Techniques for
measuring protein fractions in feedstuffs. Proc. Cornell Nutr.
Conf., p. 81-88, Ithaca, NY.
SniffenC J, O'Connor J D, Van Soest P J, Fox D G and
Russell J B 1992 A net carbohydrate and protein system for
evaluating diets: II. Carbohydrate and protein availability. J.
Anim. Sci. 70, 3562-3577.
Van Soest P J 1994 Nutritional Ecology of the Ruminant.
2nd edn., Cornell University Press, Ithaca, NY, pp.
476.
Van Soest P J, Robertson J B and Lewis B A 1991 Methods
for dietary fiber, neutral-detergent fiber and nonstarch
polysaccharides in relation to animal nutrition. J. Dairy Sci., 74:
3583-3597.
Van Soest P J and Mason V C 1991 The influence of the
Maillard reaction upon the nutritive value of fibrous feeds. Anim.
Feed Sci. Technol. 32: 45-53.
Vanzant E S, Cochran R C, Titgemeyer E C, Stafford S D,
Olson K C, Johnson D E and Jean G St 1996 In vivo and in situ
measurement of forage protein degradation in beef cattle. J. Anim.
Sci. 74, 2773-2784.
Zhang Weixian, Gu Chuan Xue, Dolberg
F and Finlayson P M 1994 Supplementation of ammoniated wheat straw with
hulled cottonseed cake. Livestock Research for Rural Development
(6) 1. http://www.cipav.org.co/lrrd/lrrd6/1/china1.htm.