Study on ruminal degradability of local plants by using nylon bag technique
Suchitra Kanpukdee and Metha Wanapat
Tropical Feed Resources Research and Development Center, Department of
Animal Science, Faculty of Agriculture, Khon Kaen University, Khon Kaen
40002, Thailand.
nunim_su@yahoo.com,
Abstract
Two, ruminally fistulated crossbred beef steers of 400±15 kg BW were used to evaluate the nutritive value of local plants by using the in sacco nylon bag technique. A Randomized Complete Block Design (RCBD) was used to determine ruminal degradability of DM and OM, and their effects on rumen ecology in cattle. The investigation was carried out with eleven local plants, namely: mangosteen (Garcinia mangostana) peel of fruit (MSP), guava (Psidium guajava) leaf (GVL), siam neem tree (Azadirachta indica) leaf (SNTL), sesbania (Sesbania grandoflora) leaf (SBNL), coral leaf (Eritrina variegate) (CRL), Bai Yanang (Tiliacora triandra) (BY), cassava hay (Manihot esculenta, Crantz) (CH), bitter cucumber (Mormormdica charantia) fruit (BCF), banana (Musa sapientum) leaf (BNL), mulberry (Morus indica) leaf (MBL), and Plia farn (Macropanax dispermus Ktze) leaf (PFL). Approximately 5 g of feed samples were weighed into duplicated nylon bags (38 µm pore size) and incubated ruminally at 0, 2, 4, 8, 12, 24, 48, and 72 h-post feeding.
The results showed that the mean values of ruminal pH (6.6) and temperature (38.8 ºC) were not different (P>0.05) among different times of incubation. The condensed tannins (CT), and crude saponin (CS) values of local plants were 15.8, 9.8 % for MSP, 14.8, 2.8 % for GVL, 11.0, 2.5 % for SNTL, 4.0, 2.0 % for SBNL, 2.3, 1.8 % for CRL, 2.2, 1.7 % for CH, 2.2, 1.3 % for BY, 2.1, 4.1 % for BCF, 2.0, 1.4 % for PFL, 1.7, 1.3 % for BNL, and 1.6, 2.3 % for MBL, respectively. The highest and lowest of the potential degradability (a+b), and effective degradability of DM and OM of feed sources for PFL and MSP were 97 and 72.3, 98.1 and 73.2, 58.6 and 46.7 and 59.1 and 45.7 %, respectively. It was also shown that PFL had a higher (p<0.01) degradability in the rumen, while MSP had the lowest, thus resulting in higher rumen degradable and undegradable roughage sources, respectively.
Based on these results PFL can be used efficiently in the rumen and MSP as a rumen by-pass protein due to its CT and CS contents.
Key word: Rumen degradability; In sacco technique; Local plants feed resources; Ruminants; Nylon bag; Digestibility
Introduction
Ruminant diets in most developing countries are based on fibrous feeds and crop residues. These feeds are imbalanced and are particularly deficient in protein, minerals, vitamins, and are highly lignified. Efficient supplementation of locally mixed concentrate with grains or protein foliages has been demonstrated to improve rumen ecology, dry matter intake and subsequently meat and milk quantity and quality (Wanapat, 1999). Tree leaves have high protein contents (18-26% crude protein), and some of them have low rates of degradability in the rumen (Espinosa, 1984). These characteristics, along with those mentioned above, make them an alternative source of by-pass protein to be used as a supplement for ruminant production systems in the tropics. The extent to which tree foliage protein is degraded in, or escapes from the rumen is extremely important. If the tree foliage protein is totally degraded, it provides ammonia and minerals for microbial growth (Leng, 1993). Local feed resources such as cassava root/hay/silage, corn stovers, kapok meal, baby corn, cow-pea, cotton seed meal, leuceana leaves, sweet potatoes, sugarcane, sesbania seed/leaves, mulberry leaves, moringa seed, sapindus fruit etc, have potential as ruminant feeds to improve and increase the efficiency of the production system (Liu et al, 2001; Preston, 2001; Hossain and Becker, 2002; Hess et al., 2003; Lam, 2003; Promkot and Wanapat, 2003; Vongsamphanh, 2003; Wanapat, 2003; Anhwange et al., 2004; Hristove et al., 2004). However, some of these feeds contain secondary plant compounds such as condensed tannins, saponins, gossypol, mimosine, and trypsin inhibitor, which may diminish the effects of these feedstuffs with respect to feed quality and animal production.
Comprehensive
reviews on the effects of secondary compounds and detoxification methods on
animal nutrition and feeding in the tropics have been reported by Reed
(1995), Abdullah and Rajion, (1997); Makkar and Becker, (1999). Limited
information is available on characteristics of DM and OM degradation in the
rumen of feed resources locally used for livestock in the tropics with
special reference to Thailand eg. mangosteen (Garcinia mangostana), peel of
fruit, guava (Psidium guajava) leaf, siam neem tree (Azadirachta
indica) leaf, sesbania (Sesbania grandoflora) leaf, coral leaf (Eritrina
variegate), Bai Yanang (Tiliacora triandra), etc. These
feeds contain high levels of condensed tannins and/or crude saponins and can
be used as alternative dietary strategic supplements to improve rumen
ecology and act as defaunating sources in ruminants. Therefore, the
objective of this study was to determine DM and OM disappearance of tropical
feeds of eleven locally available feeds in Thailand on fermentation
characteristics using the in sacco technique.
Materials and methods
Location and duration
This experiment was conducted on station at the Tropical Feed Resources Research and Development Center (TROFREC), Department of Animal Science, Faculty of Agriculture, Khon Kaen University, Thailand.
Experimental design
A Randomized complete block design (RCBD) with eleven treatments and two replicates per treatments was used.
Feed samples
Eleven local plants were used as substrates in this experiment. Most of them were collected from nearby and some were bought in local markets. All samples were dried in a forced air oven at 60 oC and ground to pass a 1 mm screen and stored for chemical analysis and the degradability study. The local plant materials investigated were as follows:
1. Mangosteen (Garcinia mangostana), Peel of fruit
2. Guava (Psidium guajava), Leaf
3. Siam neem tree (Azadirachta indica), Leaf
4. Sesbania (Sesbania grandoflora), Leaf
5. Coral leaf (Eritrina variegate)
6. Bai Yanang (Tiliacora triandra)
7. Cassava hay (Manihot esculenta Crantz)
8. Bitter cucumber (Mormormdica charantia), Fruit
9. Mulbery (Morus indica), Leaf
10. Banana (Musa sapientum), Leaf
11. Plia farn (Macropanax dispermus Ktze), Leaf
Animal and diets
Two ruminally fistulated crossbred beef steers with liveweight 400±15 kg were used as replicates to determine in sacco DM and OM degradability of eleven local plants. Steers were housed in individual pens and fed ad libitum urea-treated rice straw (UTRS) and concentrate (12% CP) at 70:30 ratio. Water and mineral block were available at all times. The diets were offered in two equal meals at 07.00h and 16.00h. The animals were adapted to the basal feed for two weeks prior to suspension of the bags.
Ruminal disappearance study
The DM and OM disappearances in the rumen were estimated for each feed sample using the nylon bag technique (Ørskov and McDonald 1979). The bags (7x 14cm) were made from dacron cloth with a pore size of 38 μm. Approximately 5.0 g of dried (60ْ C) feed samples were weighed into previously tired the nylon bags. The bags were tied to weight chains and place in ventral rumen sac of steers approximate 2 h after the morning feeding. All feed samples were incubated simultaneously in both steers using duplicates base at each time point and a blank bag containing no sample for each removal time. Bags for each feed sample were removed after 0, 2, 4, 8, 12, 24, 48 and 72 h of incubation. Immediately after removing from the rumen, the bags were washed with cold tap water until clear and dried in forced air oven at 60 ο C for 72 h. The bags were weighed and residues were removed and then analyzed for DM and OM. All bag feed samples were collected for their corresponding blank. The 0 h incubation samples were washed and dried in similar conditions. The bags were weighed and tested according to the procedure described by Ørskov and McDonald (1979). Samples of rumen fluid were taken through the ruminal canulae at 0, 2, 4, 8, 12, 24, 48 and 72 h of incubation, during each time, and pH and temperature were measured immediately using a portable pH and temperature meter.
Chemical composition analysis
The samples were analyzed for DM, Ash, and CP according to AOAC (1990). Neutral-detergent fiber (NDF) and acid-detergent fiber (ADF) were determined using the method of Van Soest et al. (1991). Condensed tannins (CT) were estimated by the Vanillin-HCL method (Burns, 1971 modified by Wanapat and Poungchompu, 2001) and saponins were measured by using methanol extraction following the method of Kwon et al. (2002) as modified by Wanapat and Ngamsaeng (2004).
Data analysis
Data for ruminal disappearance characteristics of DM and OM were fitted to the exponential equation following the procedure described by Ørskov and McDonald (1979) and using the NEWAY program (Chen, 1996). P = a+b (1-e-ct) where, P = disappearance rate at time t (%), a = the intercept of the degradation curve at time zero (%), b = the fraction of DM and OM which were degraded when given sufficient time for digestion in the rumen (%), c = a rate constant of disappearance of fraction b (h-1), and t = time of incubation (h). The effective degradability (ED) of DM and OM were calculated by using the following equation. EDDM or EDOM = a+{(bc)/(c+k)} where, k = assuming the rate of particulate outflow from the rumen, k, is 0.05 h-1 by equation of Ørskov and McDonald (1979)
Statistical analysis
Data were analyzed by Analysis of variance (ANOVA) according to a Randomized complete block design (RCBD). It was performed on the data of the same incubation time as a separate set following the ANOVA procedure of SAS (1998). Treatment means were compared using Duncan's New Multiple Range Test (Steel and Torrie, 1980). The statistical model was: Yij = m + δi+Tj +eij where; Yij = Observation in block i and treatment j, m = Over all sample mean, δi = Block i, Ti = Effect of treatment i, eij = Error
Results
Chemical composition of feedstuffs
The chemical composition of feedstuffs is presented in Table1. All eleven feed sources had similar DM and OM contents. The CP content ranged from 2.9 % in bitter cucumber to 30.8 % in sesbania leaf. More than half of the samples had CP, NDF and ADF contents ranging from 12.2 to 21.5%, 25.4 to 73.8% and 12.6 to 50.0%, respectively. Feed sources could be divided into three groups: high, medium, and low depending on CT concentration, which ranged from 11.4 to 15.8%, 2.1 to 4.6% and 1.6 to 1.7%, respectively. MSP had the highest and MBL the lowest CT values, while BY and BNL had the lowest CS values and MSP had the highest (1.3 % and 9.8 %, respectively).
|
Table 1. Chemical composition of local feeds resources used in in sacco technique |
||||||||
|
Substrates |
DM |
Ash |
OM |
CP |
NDF |
ADF |
CT1 |
CS2 |
|
% in DM |
||||||||
|
Mangosteen peel |
95.3 |
2.6 |
97.4 |
21.5 |
52.5 |
50.0 |
15.8 |
9.8 |
|
Guava leaf |
94.7 |
7.7 |
92.3 |
14.0 |
55.0 |
32.6 |
14.8 |
2.8 |
|
Siam neem tree leaf |
94.8 |
10.8 |
89.2 |
13.3 |
50.8 |
33.9 |
11.0 |
2.5 |
|
Sesbania leaf |
94.9 |
8.0 |
92.0 |
30.8 |
29.4 |
15.6 |
4.0 |
2.0 |
|
Coral leaf |
95.0 |
10.4 |
89.6 |
23.0 |
48.9 |
29.9 |
2.3 |
1.8 |
|
Bai yanang leaf |
93.1 |
6.8 |
93.2 |
17.1 |
60.2 |
38.1 |
2.2 |
1.3 |
|
Cassava hay |
93.2 |
7.0 |
93.0 |
25.4 |
52.3 |
29.8 |
2.2 |
1.7 |
|
Bitter cucumber fruit |
94.2 |
9.6 |
90.4 |
2.9 |
52.5 |
31.6 |
2.1 |
4.1 |
|
Banana leaf |
96.2 |
7.8 |
92.2 |
14.8 |
73.8 |
34.8 |
1.7 |
1.3 |
|
Mulberry leaf |
95.4 |
12.8 |
87.2 |
17.2 |
55.2 |
22.5 |
1.6 |
2.3 |
|
Plia fran leaf |
95.5 |
5.1 |
94.9 |
12.2 |
28.2 |
13.6 |
2.0 |
1.4 |
|
DM = dry matter, OM = organic matter, CP = crude protein, NDF = neutral-detergent fiber, ADF = acid-detergent fiber, CT1 = Condensed tannins; CS2 = Crude saponins |
||||||||
Ruminal environment
Rumen environment expressed by the level of pH and temperature is shown in Table 2. The average ruminal pH and temperature was 6.6 and 38.8 ºC, respectively. There were no differences of these values among times of incubation.
|
Table 2. Ruminal pH and temperature in beef steers during nylon bag study |
||
|
h-post suspension |
pH |
Temperature (ºC) |
|
0 |
6.6 |
38.8 |
|
2 |
6.7 |
38.4 |
|
4 |
6.6 |
38.4 |
|
8 |
6.6 |
38.9 |
|
12 |
6.6 |
38.4 |
|
24 |
6.7 |
39.0 |
|
48 |
6.6 |
39.5 |
|
72 |
6.8 |
38.5 |
|
Mean ± SD |
6.6 ± 0.1 |
38.8 ± 0.4 |
Ruminal DM and OM disappearance and characteristics
Ruminal DM and OM disappearance and characteristics of ruminal DM and OM disappearance of the eleven feed sources are shown in Figures 1, 2, and Table 3, respectively. Ruminal DM and OM disappearances increased with rumen incubation time for all feed sources (0 to72 h). PFL had the greatest degradability in the rumen, while MSP was lowest on DM and OM degradability after suspension in the rumen from 12 to 72 h. However, after suspension of GVL from 2 to 12 h, and for BY from 2 to 8 h in the rumen, the values of DM and OM degradability were lower than that of MSP. DM and OM degradability of feed sources can be divided into three groups, depending on their degradability in the rumen. PFL and SBNL had a high rapid rumen degradability, while BCF, MBL, CH, and SNTL had a medium rumen degradability; CRL, BY, BNL, GVL, and MSP had the lowest rumen degradability (Figure 1 and 2). The amounts of DM and OM degraded after 12 h in sacco were more than 50 % of the total which had been found in SBNL, PLF, CH, MBL, SNTL, BCF, CRL, BY and BNL respectively, except for MSP and GVL.
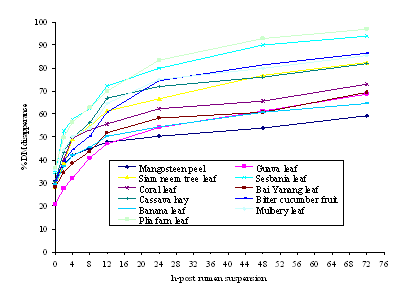
|
| Figure1. In sacco dry matter (DM) disappearances of feedstuffs. |
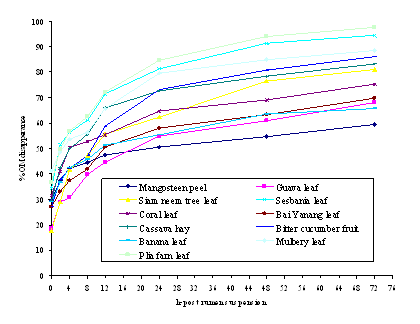
|
| Figure2. In sacco organic matter (OM) disappearances of feedstuffs. |
The amount of DM and OM disappearance and characteristics ranked from the highest to the lowest degradation rate (c) were as follows: CH, BY, MBL, CRL, BCF, SNTL, GVL, PEL, SBNL, BNL, and MSP, respectively. DM and OM degradation rate constants (c) of CH was significantly highest (p<0.01) as compared with the other feed sources, while BY, MBL, CRL, BCF, SNTL, GVL, PFL, SBNL and BNL were degraded to similar extents. The degraded fraction (a) of DM and OM was significantly highest (p<0.01) in SBNL and lowest in GVL (48.7 and 24.1, respectively). The insoluble degradability fractions (b) and the potential degradability (a+b) of DM and OM were highest (P<0.01) in BCF and PFL (53.6, 55.4, 97.9 and 98.1, respectively), as compared to the other feed sources. Similarly, effective degradability of DM and OM at outflow rate of passage of 0.05/h-1, PFL (72.3 and 73.2 respectively) was higher than the other feed sources.
|
Table 3. Dry matter (DM) and organic matter (OM) disappearances of local plant feedstuffs incubated in the rumen at various times in beef cattle |
||||||||||||
|
Item |
PFL2/ |
SBNL |
MBL |
CH |
BCF |
SNTL |
CRL |
BY |
BNL |
GVL |
MSP |
SEM |
|
a |
45.4a |
48.7b |
39.2cd |
36.9ed |
33.0f |
37.6ed |
40.3c |
30.8g |
35.9e |
24.1h |
38.2cde |
1.13 |
|
b |
52.5a |
45.9b |
45.5bc |
42.6d |
53.6a |
43.6cd |
30.8f |
36.2e |
28.7g |
42.8d |
20.4h |
1.03 |
|
c |
0.052a |
0.051a |
0.061a |
0.086b |
0.058a |
0.058a |
0.059a |
0.061a |
0.050a |
0.056a |
0.043a |
0.01 |
|
a+b |
97.9a |
94.7b |
84.7c |
79.5d |
86.6c |
81.2d |
71.1e |
67.0f |
64.6g |
66.9f |
58.6h |
1.06 |
|
1/EDDM,% |
72.3a |
71.8a |
64.2b |
63.6b |
61.7c |
60.8c |
56.7e |
50.5f |
50.2f |
47.6g |
46.7h |
0.40 |
|
a |
44.6ab |
46.5a |
43.2b |
37.2d |
31.2f |
27.8g |
40.9c |
29.1g |
34.8e |
22.1h |
37.6d |
0.99 |
|
b |
53.5a |
49.1b |
46.0c |
44.4c |
55.4a |
53.1a |
33.5e |
39.6d |
31.3e |
44.6c |
21.5f |
1.13 |
|
c |
0.058a |
0.053a |
0.056a |
0.076b |
0.055a |
0.055a |
0.051a |
0.056a |
0.055a |
0.056a |
0.043a |
0.01 |
|
a+b |
98.1a |
95.6b |
89.2c |
81.6e |
86.6d |
80.9e |
74.3f |
68.6g |
66.1h |
66.7gh |
59.1i |
0.99 |
|
1/EDOM, % |
73.2a |
71.6b |
67.4c |
63.8d |
60.0e |
55.3g |
57.7f |
49.9i |
51.0h |
47.5j |
45.7k |
0.54 |
|
a-k Means within rows not sharing a common superscript are different at P<0.01 1/Effective degradability in the rumen (assuming rate of passage of 0.05/h-1), SEM = Standard error of the mean EDDM = effective degradability of dry matter, EDOM = effective degradability of organic matter 2/ PFL = Plia farn leaf, SBNL = sesbania leaf, MBL = mulbery leaf, CH = cassava hay, BCF = bitter cucumber fruit, SNTL = siam neem tree leaf, CRL = coral leaf, BY = Bai Yanang leaf, BNL = banana leaf, GVL = guava leaf , MSP = mangosteen peel |
||||||||||||