Utilization of Fly larvae, Earthworms and Duckweed
for Frog production in an Integrated Farming System
Latsamy Phounvisouk
LivingAquatic Resources Research
Center (LARReC)
NaongThang Villged, Vientiane City, Laos
PDR
Meetouna@yahoo.com
1. Introduction
Livestock excreta are a major source of environmental pollution
in intensive, specialized animal agriculture (Steinfeld et
al., 2006). According to Kirschenmann (2007)
"Agro-ecologists increasingly are convinced that the most viable
alternative technology will spring from the biological synergies
inherent in multispecies systems and that additionalresearch might
make such systems the next new technology". Integration of crops
and livestock, with recycling of the manure, is considered to be
the basis of new management systems, "with the potential to
increase the quantity and quality of production and the economic
return to the farmers while at the same time placing less degrading
pressure on soil and water resources." (Franzluebbers, 2007). One
way of improving animal production without negative effects on the
environment and increasing the efficiency of resource utilization
is by integrating all the processes in the system, in the context
of the sustainable use of those resources (Sophin and Preston,
2001).
To solve the problem of pollution, and to make better use of
animal excreta, there are several techniques that can be applied.
Biodigesters
Anaerobic biodigestion is one of the most efficient technologies
for converting animal manure into useful products: gas for cooking
and effluent for fertilizer (Preston and Rodriguez 1998). For
small-scale farms the plastic biodigester, because of its low cost
and simple construction and management, may be the preferred
technology (Bui Xuan An et al., 1997). The effluent is a
valuable product from biodigesters and has been shown to be
superior to the original manure for fertilization of crops (Le Ha
Chau 1998) and fish ponds (Pich Sophin and Preston 2001). The
effluents from biodigesters, when suitably diluted, are very
effective media for growing duckweed (Le Ha Chau
1998).
Earthworms
The cultivation of earthworms can be another component of manure
recycling. They have a comparative advantage over other forms of
recycling when the manure is from goats or rabbits (Preston and
Rodriguez 1998).
Fly larvae
Manure is the principal food of many insects in nature, house
fly (Muscadomestica Linnaeus). The larvae convert
residual manure proteins and other nutrients into their biomass,
which is rich in high quality animal protein. Also, while occupying
the manure they aerate and dry it, reducing odors.
Aquatic plants
Aquatic plants are being promoted as important components in
management of livestock wastes, especially in large scale intensive
units (Steinfeld et al., 2006). According to Leng
(1995).farmers throughout Asia have harvested naturally produced
aquatic plants for a number of purposes including animal feed,
green manure and for their family feed resources. "The best known
of these is "duckweed", which has become prominent, because of its
ability to concentrate minerals in heavily polluted water such as
that arising from sewage treatment facilities"(Leng 1995). It has
also high potential as a feed resource for fish (Leng et
al., 1994), ducks (Bui Xuan Men et al., 1997) and pigs
(Du Thnh Hang 1998). Duckweed grows on water with relatively high
levels of N, P and K and concentrates the minerals and synthesises
protein.
Potential of frog cultivation in waste recycling
Live stock play two roles in waste recycling. On the one hand
they maybe the cause of pollution if their manure is discharged
directly to the environment. However, certain species may be able
to benefit directly from products that arise in an efficient system
of waste recycling. Aquatic animals are most suited for the
utilization of products of recycling since their normal habitat is
either wholly or partially in natural or artificial water bodies.
The common lowland frog (Rana rugulosa) appears to
be specially adapted for cultivation in integrated farming systems,
since its natural feed source includes insects and earth worms. In
the Northern region of Laos, there is a high demand for consumption
in family households and local markets. The frog is fast growing.
From hatching to market size, it takes 3 to 4 months which is
similar to other commercial species such as catfish and tilapia.
Frogs can be raised in all locations in Laos, as they require small
area and lower quantity of water than other aquaculture species
(Bounsong, 2001).
2.
Objectives
The aims of the present study were to investigate components in
a waste recycling system which could be used for the cultivation of
frogs, to reduce the present dependency on imported concentrate
feeds, and to make the system more attractive for small-scale
farmers in Laos.
Two experiments have been carried out. The first experiment was
a study of the effect of different sources of household waste,
irrigated with biodigester effluent, on the production of fly
larvae. The second experiment compared the use of fly larvae and
earth worms, with or without duck weed, as the basal diet for
growing frogs raised in ponds.
Table of contents
3.1. Biological characteristics and
feeding behaviour of frog species. 9
3.1.1. The common lowland frog,
(Rana Rugulosa)9
3.1.5. Nutrient Requirements.
10
3.2. Frog production in Aquaculture
systems. 10
3.3. Earthworm as a feed source for
livestock and frog production. 11
3.3.1. Significance of Earthworm in
animal production. 11
3.3.2. Feed and feeding of
Earthworm.. 11
3.3.3. Nutritive value of
earthworms. 11
3.4. Nutritive value of fly
larvae. 12
3.4.1. Larvae as a feed source for
livestock production. 13
3.4.2. Research on fly larvae in the
past13
3.4.3. Production of fly larvae.
13
3.4.4 Fly larvae and chitin.
14
3.5. Biodigesters in integrated
farming systems. 17
3.7. Water quality parameters for
Frogs. 18
4. Conclusions and recommendations.
19
3.
General discussion
3.1. Biological characteristics and feeding
behaviourof frog species
3.1.1. The common lowland frog,
(RanaRugulosa)
The local lowland frog, Rana rugulosa Wiegmann,
locally called "Kob Thong" or "Kob Jarn" is one of 38 frog species
found in Laos. At present, four native and one introduced species,
RanaRugulosa, Rana tigerina, Rana marcrodon, Rana
blythii and Rana catesbeiana (bullfrog),
respectively, are most preferred in both local and foreign markets.
Because of a high demand, frog farms continue to expand throughout
the SE Asia region (Thongyount, 2004).
3.1.2. .Natural
habitat
The lowland frog is generally found in various water bodies and
wet or damp areas. They can be found in a considerable distance
from their aquatic habitat because of their wandering
behavior.
3.1.3. Food and
feeding
The lowland frog (Rana rugulosa)is a carnivorous
amphibian, feeding on earthworms, insects, spiders, worms, small
fish and small frogs (Akasay, 1994). In their natural habitat,
frogs have a variety of foods to select except at times of
unfavorable environment or seasonal conditions. In Thailand, frog
culture is usually conducted in concrete tanks, floating net cages,
or in ponds with commercial feed (Kamonphone, 1977).
3.1.4. Stocking
density
After they are taken from tadpole ponds, young frogs can be
stocked in culture pens or concrete tank at up 30 frogs per square
meter (Grey Lutz and Jimmy, 1999).
3.1.5. Nutrient
Requirements
The lowland frog require similar nutrients as other aquatic
animal species, especially those that are carnivorous, to maintain
normal growth and metabolic function. The major nutrients such as
protein, lipids, essential fatty acids, carbohydrates, vitamins and
minerals are required for normal growth (LARReC,
2001).
The dietary protein requirements for frogs have been determined
in various studies. According to Thongyount (2004), protein levels
start at 38% in DM for tadpoles (1-30 days), reducing to 32% for
small frogs (30-60 days) and 26% for fattening frog (60-90 days).
Artificial feed of 35 % crude protein supported faster growth in
Rana pipen tadpoles than young tilapia fish of 23 %
protein (Ling et al., 2003). Feeding of ensiled fish, over a
period of 4 months, enabled frogs to reach a live weight of 200 to
400g (Marttinez, 1993). According to Martinez et al., (1993)
commercial feeds with 39% protein feed are suitable for frogs in
intensive culture.
3.2. Frog production in Aquaculture
systems
In general, there are three forms of frog culture: in ponds,
cages and tanks. Each system has its own important characteristics
and the decision as to which system to use will depend on the
purpose of the operation. Cage and tank culture are the preferred
systems for commercial production. However, for the experiment
described in Paper II, earthen ponds lined with plastic sheets were
used as this facilitated establishing the required degree of
replication of the treatments.
3.3. Earthworm as a
feed source for livestock and frog production
Contribution of earthworm to our ecosystem is often
underestimated. Not only improving degradation rate of the waste,
they could be used as animal feed as containing high protein
content and many important amino acids (Parlevliet 1997).
3.3.1. Significance of Earthworm in animal
production
Earthworm can be used as feed ingredient for fish, poultry,
amphibians and pigs. They can be mixed with other feed ingredients
such as rice brume, broken rice and others (Samphone et al.,
1999). Such mixed feed ensure rapid growth of animals which can
tolerate hash environmental conditions and diseases Parlevliet
(1997).
3.3.2. Feed and feeding of
Earthworm
Earthworms live in holes they made in the soil. Earthworm intake
food through the mouth and discharge through the other end the
holes in the soil are very small in size and folds themselves in
the holes. They normally do not appear on the surface during day
time except when there is a heavy rain. Usually Earthworm can be
found on soil surface at night especially after the rain. When the
weather is dry and cold digs their holes as deep as 2.5 meters, and
under such environmental condition, Earthworm will twist themselves
around one another to form a crump and release sticky liquid
substance. At night Earthworm will feed on plant materials on the
surface and return to their holes after discharging waste materials
close to their holes (Vineset et al., 1997).
3.3.3. Nutritive value of
earthworms
Sun-dried earthworms, Perionyx excavatus, has
93.62% dry matter, 59.90% crude protein, 402.09 Kcal/100 g gross
energy, 7.43% fatty acid, 7.43% crude fibre, 1.73% Ca, and 0.118% P
(Bay, 2002). Earthworm meal is very high in protein, and with
variable oil content (Table 1). The dry matter content of
earthworms ranges between about 15 and 20%. The fatty acid
composition of the lipid extracted from worms is quite similar to
the lipid composition of some fish oils, being high in φ-3
polyunsaturated lipids. In our study natural earthworms had average
protein (70% in dry matter (DM)) and fat (13 % in DM) contents.
However, they have a comparative advantage over other forms of
recycling when the faeces are from goats or rabbits (Nguyen Quang
Suc et al., 2000). Recently, the use of the earthworm
(Eisenia foetida) as an agent for recycling live
stock manure has received increasing attention (Preston and
Rodríguez, 2002).
|
Table 1. Chemical composition of earthworms (g/kg DM basis, except for DM which is on fresh basis) |
||||||||
|
|
DM |
Crude |
Organic matter |
Crude fat |
Crude fibre |
Ash |
Ca |
P |
|
Earthworm |
214 |
572 |
952 |
79.4 |
11.2 |
48.1 |
14.5 |
7.0 |
|
DM = dry matter, Ca = calcium, P
= phosphorous |
||||||||
|
Table 2. Amino acid content in earthworm (P. excavatus) (g/16g N) |
||
|
|
(Tram et al 2005) |
(Bay 2002) |
|
Aspartic |
5.56 |
6.51 |
|
Glutamic |
12.47 |
12.64 |
|
Serine |
4.28 |
4.15 |
|
Histidine |
5.05 |
5.26 |
|
Glycine |
3.86 |
2.55 |
|
Threonine |
2.18 |
2.58 |
|
Alanine |
3.25 |
2.8 |
|
Arginine |
6.36 |
10.83 |
|
Tyrosine |
4.48 |
5.96 |
|
Valine |
4.65 |
8.62 |
|
Methionine |
2.1 |
1.92 |
|
Phenylalanine |
2.16 |
2.67 |
|
Isoleucine |
4.79 |
8.14 |
|
Leucine |
6.06 |
7.72 |
|
Lysine |
3.58 |
3.48 |
|
Hydroxyproline |
4.24 |
- |
|
Proline |
3.17 |
2.65 |
3.4. Nutritivevalue of
fly larvae
House fly (Muscadomestica) larvae has 30% DM and, in the DM, 63% protein and 15% fat according to Newton et al., (1977). By contrast, in Paper 11 it was found that the protein was lower (49% in DM) and the fat much higher (31% in DM). Due to the protein content, the fly larval meal is considered to be a suitable replacement for conventional protein and fat sources. Comparative amino acid profiles of the proteins of fishmeal and house fly larvae are in Table 3.
|
Table 3. Comparative amino acid profile of the proteins of fishmeal and house fly larvae (g /16g N) |
||
|
|
Fish meal |
Fly larvae |
|
Alanine |
6.34 |
6.15 |
|
Arginine |
5.82 |
5.42 |
|
Aspartic |
9.35 |
10.8 |
|
Cystine |
0.70 |
0.82 |
|
Glutamic |
13.3 |
12.2 |
|
Glycine |
5.90 |
5.40 |
|
Histidine |
2.22 |
3.50 |
|
Isoleucine |
4.85 |
4.13 |
|
Leucine |
7.35 |
6.95 |
|
Lysine |
7.85 |
7.37 |
|
Methionine |
2.84 |
2.24 |
|
Phenylalanin |
4.35 |
6.95 |
|
Proline |
4.35 |
3.66 |
|
Serine |
4.55 |
4.51 |
|
Threonine |
4.55 |
4.53 |
|
Tryptophan |
1.33 |
1.45 |
|
Tyrosine |
3.45 |
8.10 |
|
Valine |
5.65 |
5.60 |
|
Source: Spinelli No date |
||
3.4.1. Larvae as a
feed source for livestock production
Fly larvae (Mosca spp) have been fed experimentally to
several animals, with larvae or prepupae used to replace soybean or
fish meal in a formulated diet (Sheppard. No. date). Fly larvae
feeding tests have utilized cockerels (Hale, 1973), pigs (Newton
et al., 1977) and catfish (Bondarie et al, 1987). The
larvae have been shown to be generally equal to soybean meal (and
other conventional ingredients) in feed value when fed to chicks
(Calvert et al., 1970).
3.4.2. Research on fly larvae in the
past
Complete replacement of the protein supplemented by fresh
termites or fly larvae produced on farm from waste bagasse, wood or
fresh pigs manure, and managed and harvested by household labour,
decreased the chicken feed costs by 40% and 51% respectively,
compared to the control diet (Men, 2005).
3.4.3. Production of fly
larvae
Viet Chuong (2001) investigated a production of fly larvae from
a rectangular holes, 50 x 50 cm and deep which dug and filled with
soft, damp rice straw and fresh cattle manure in alternate 15 cm
thick layers. A thin layer of rice gruel was placed on the top
straw layer. Around one kg of spoilt fish was added to attract
flies to lay their eggs, and small shelters were constructed over
the holes to protect them from rain and direct sunlight, and to
allow the flies to enter the hole easily. To maintain high humidity
2-3 litres of water were poured over the holes when the eggs had
been laid. Growing larvae were harvested 4-5 days after putting the
bait into the holes, the yields of larvae harvested after the 5
days period were very low. It was found that this method is not
suitable for producing fly larvae under these particular
experimental conditions.
3.4.4
Fly larvae and chitin
Chitin is the most widespread amino polysaccharide in nature and
is estimated annually to be produced almost as much as cellulose.
It is mainly found in arthropod exoskeletons, fungal cell walls or
nematode egg shells. However, derivatives of chitin oligomershave
also been implicated as morphogenic factors in the communication
between leguminous plants and Rhizobium and even in
vertebrates, where they may be important during early stages of
embryogenesis. Chitin is one of the most important biopolymers in
nature. It is mainly produced by fungi, arthropods and nematodes.
In insects, it functions as scaffold material, supporting the
cuticles of the epidermis and trachea as well as the peritrophic
matrices lining the gut epithelium.
Chitin is composed largely of alternating
Nacetylglucosamine residues, which are linked by b-(1-4)-
glycosidic bonds. Since hydrolysis of chitin by chitinase treatment
leads to the release of glucosamine in addition to
Nacetylglucosamine, it was concluded that glucosamine might
be a significant portion of the polymer. However, solids NMR
analysis of tobacco hornworm cuticle preparations suggested that
little or no glucosamine is present (Kramer et al., 1995). Chitin
polymers tend to form microfibrils (also referred to as rods or
crystallites) of ~3·nm in diameter that are stabilized by
hydrogen bonds formed between the amine and carbonyl groups. Chitin
microfibrils of peritrophic matrices may even exceed 0.5·mm in
length and frequently associate in bundles containing parallel
groups of 10 or more single microfibrils (Peters et al., 1979;
Lehane, 1997). X-ray diffraction analysis suggested that chitin is
a polymorphic substance that occurs in three different crystalline
modifications, termed a-, b- and g- chitin. They mainly differ in
the degree of hydration, in the size of the unit cell and in the
number of chitin chains per unit cell (Rudall and Kenchington,
1973; Kramer and Koga, 1986). In the a form, all chains exhibit an
anti-parallel orientation; in the b form the chains are arranged in
a parallel manner; in the g form sets of two parallel strands
alternate with single antiparallel strands. In addition,
non-crystalline, transient states have also been reported in a
fungal system (Vermeulen and Wessels, 1986). All three crystalline
modifications are actually found in chitinous structures of
insects. The a form is most prevalent in chitinous cuticles,
whereas the b and g forms are frequently found in cocoons
(Kenchington, 1976; Peters, 1992). Peritrophic matrices usually
consist of a- and b-chitin. Sometimes the presence of b-chitin in
cocoons is traced back to the fact that some cocoons are formed
from peritrophic matrices; for example, those of Australian spider
beetle Ptinus tectus, a specialized beetle (Rudall
and Kenchington, 1973). The anti-parallel arrangement of chitin
molecules in the a form allows tight packaging into chitin
microfibrils, consisting of ~20 single chitin chains that are
stabilized by a high number of hydrogen bonds formed within and
between the molecules. This arrangement may contribute
significantly to the physicochemical properties of the cuticle such
as mechanical strength and stability (Giraud-Guille and Bouligand,
1986). By contrast, in the b- and g-chains, packing tightness and
numbers of inter-chain hydrogen bonds are reduced, resulting in an
increased number of hydrogen bonds with water. The high degree of
hydration and reduced packaging tightness result in more flexible
and soft chitinous structures, as are found in peritrophic matrices
or cocoons. The picture drawn above is certainly oversimplified and
does not explain the physicochemical properties of cuticles and
peritrophic matrices adequately because it is reduced to only one
component of a complex structure. However, differences in the
arrangement of chitin microfibrils between cuticles and peritrophic
matrices may help to understand their function. The cuticle is
secreted in the form of thin layers by the apical microvilli of
epidermal cells. The chitin microfibrils are embedded into the
protein matrix and stabilize it in a way that resembles
constructions of steelreinforced concrete. Since horizontal
microfibrils, in parallel with the cuticle plane, rotate either
progressively or abruptly from one level to another, complex
patterns (e.g. helicoidal) and textures (e.g. plywood-like
structures) arise, depending on the degree of rotational
displacement (Bouligand, 1972). By contrast, in peritrophic
matrices, the microfibrils are normally arranged as a network of
randomly organized, felt-like structures embedded in an amorphous
matrix, and only in a few cases have higher ordered configurations
been reported (Lehane, 1997).
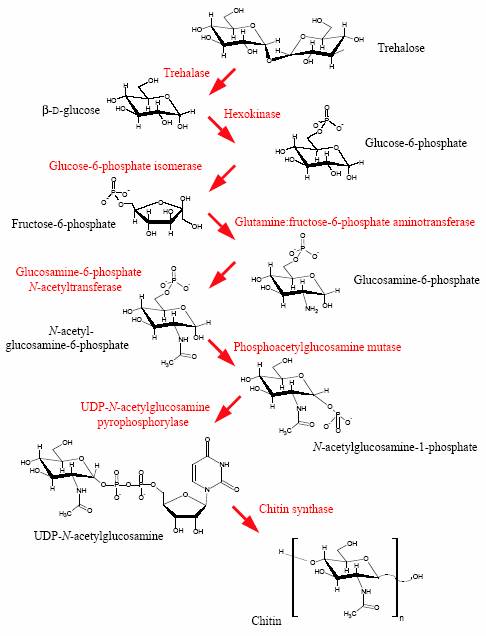
Figure.1. Biosynthesis of chitin in insects. The pathway starts
with trehalose, the main hemolymph sugar in most insects, and ends
with the chitin polymer. The diagrammatic representation is based
on previously published pathways (Kramer and Koga, 1986; Cohen,
2001).
Chitin, an integral part of the invertebrate cuticle
(exoskeleton), can be estimated by determining the acid detergent
fiber fraction corrected for ash. Since chitin contains about 7%
nitrogen, each 1% of ADF (presumed to be chitin) contains the
equivalent of 0.4% crude protein (1 x 0.07 x 6.25). It has been
reported, that some insectivores have an intestinal chitinase,
while others may rely on chitinases produced by gut microorganisms.
Chitin digestibility in three species of mammals has been shown to
range from 2-20%. However, there is no evidence that the nitrogen
released can contribute to the protein available for absorption by
the insectivore (Bernard et al., 1997).
Assuming the chitin content of the fly larvae in Paper 2 was
25%, then the protein present in the chitin would be about 6%. This
would reduce the available protein from 49% of the DM to 43% which
is still above the estimated requirements of 37% in DM.
3.5. Biodigestersin
integrated farming systems
Biodigester technology for converting manure into methane for
fuel is neither new nor uncommon. In many parts of Asia, Central
America and Europe, biodigester use is widespread. Biodigesters are
installed in these areas in response to organic waste (manure)
disposal problems and/or high-energy costs (Figure
2.)
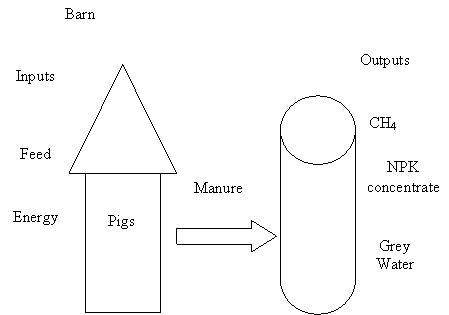
Figure.2.Biodigester Schematic
3.6. Duckweed
Conventional feed such as soya bean meal and fish meal are a
source of protein in diets for pigs and poultry. Soya bean meal and
fish meal are expensive, which results in high costs of production.
Using of local feed resources can be a way to reduce cost of
production, and as a result, increasing income. Thus, these improve
the standard of living of smallholder farmers. Duckweeds have
potential as protein sources when combined with energy-rich feeds
which are low in fiber such as broken rice, cassava root meal or
sugar cane juice (Becerra et al., 1995). Duckweed grows well
on pond surfaces. They are more resistant to pests and diseases
than other aquatic plants and have high protein and carotene
content. Protein content of duckweed responds quickly to nutrients
in a water environment (Leng et al., 1994). The effluents
from biodigesters, when suitably diluted, are very effective media
for growing duckweed (Le Ha Chau 1998). Duckweed has high crude
protein content and a well-balanced amino acid profile and is also
a good source of vitamins and minerals for livestock. Even though
the moisture content of duckweed can be a limiting factor for fish
and also frog production, duckweed can play important role in
aquatic systems. The effluent leaving the biodigester
retains the minerals and with suitable dilution is a good media for
duckweed ponds. Ponds fertilized with effluent can produce up to
100 g fresh duckweed/m²/day which is equivalent to about 6
tonnes protein/ha/year (Rodriguéz et al., no
date).
|
Table 4. Chemical composition of duckweed |
||
|
|
Ngyuyen Thi Thuy and Ogle (2005) |
Nguyen Thi Kim Khang and Ogle (2004) |
|
DM % |
5.66 |
4.7 |
|
% of DM |
|
|
|
CP |
35 |
37.3 |
|
EE |
10.5 |
9.62 |
|
CF |
6.95 |
5.85 |
|
Ca |
1.02 |
0.9 |
|
P |
1.04 |
1.5 |
|
Ash |
16.9 |
17.9 |
|
ME (MJ/kg) |
10.2 |
9.3 |
3.7. Water quality
parameters for Frogs
An abundant supply of high quality water must be readily
available to the frogs throughout the growing season. (Diana et
al., 1997) Water temperatures of 18o-22oC
are generally suitable for rearing larval salamander species found
in Laos, and temperatures of 18o-22oC are
suitable for most species of frog. Uodone (2004), the data from
frog training course at the fishery station belong to Living
Aquatic Recourse Research Center found that water quality
parameters for available to the frogs growing in Laos in (Table. 5)
suitable for most species of frog.
|
Table 5. The condition of water source for frogs growing |
||||
|
Level |
Menu |
Unit |
6 Am |
6 Pm |
|
1 |
pH value |
|
5.5 |
7.7 |
|
2 |
Dissolved oxygen |
ppm (mg/) |
5.6 |
7.2 |
|
3 |
NH3 -H |
ppm (mg/L) |
0.04 |
0.05 |
|
4 |
Water temperature |
°C |
26.5 |
27.8 |
|
5 |
Air temperature |
°C |
27.9 |
28.6 |
|
6 |
Free from Pollution |
|
|
|
|
Source: Uodone (2004) |
||||
4.
Conclusions and recommendations
Based on the results in this thesis, it is concluded
that:
-
Frog cultivation in a waste recycling system is important for sustainable aquaculture, to reduce expenditure on costly feeds.
-
Earthworms, Larvae and Duckweed could be the basis of a system to replace the high protein concentrate feed traditionally used in intensive frog culture.
-
Pig manure mixed with fermented fish waste was a better substrate for growing larvae than Jackfruit waste and pig manure.
-
There appeared to be no advantages from applying biodigester effluent to the substrates.
-
Growth rates and feed conversion (for DM and crude protein) were better, and mortality was lower, when frogs were fed earthworms rather than larvae and when they had access to fresh duckweed mixed with the larvae/earthworms.
-
The net increase in live weight (252 g in 90 days) on the best diet (earthworms plus duckweed) was better than in one report concerning frogs fed an artificial diet (200 g in 120 days).
-
Comparisons with another aquatic species (catfish) indicated broadly similar results for growth rate and feed conversion.
5.
References
AkasayN 1994 Frog culture for Commerce trade,
Cooperative Frog culture, Thailand, 10-20 p.
BouligandY 1972 Twisted fibrous arrangements in
biological materials and cholesteric
mesophases. Tissue Cell 4, 189-217
BondariK and Sheppard DC 1987 Soldier fly,
Hermetiaillucens L., larvae as feed for
channel
catfish, Ictalurus. www.p2pays.org/ref/13/12648.htm
Becerra M, Preston TR and Ogle B 1995 Effect of replacing
whole boiled soyabeans with
Lemnasp in diets of growing ducks. MSc. Thesis, Swedish
University of Agricultural Sciences.
Bernard J B, Allen Mary E and Ullrey D E 1997 Feeding
captive insectivorous animals:
Nutritional aspects of insects as food. Nutrition Advisory
Group Handbook.
BakkersJ, Kijne J W and Spaink H P 1999 Function
of chitin oligosaccharides in plant and
animal development. EXS 87,71 -83.
[Medline]
BayNV2002 Study of production and
utilization earthworms (perionyx excavatus) as feed
Supplement in chicken diet in oder to improve scavenging chicken
production system at farmers
level. Doctoral thesis. pp. 160.
Calvert C C, NO Morgan and R D Martin 1970 House fly
larvae: biodegradation of Hen
excreta to useful products.
Poultry Science, 49,
588-9.
Cohen E 2001 Chitin synthesis and inhibition: a revisit.
PestManag. Sci.57, 946-950.
Diana J S, Szyper J P, Batterson TR, Boyd C E and Piedrahita
R H 1997 water quality in
Ponds. In: Egna, H., S, Boyd, C., E (Eds), Dynamics of pond
Aquaculture. CRC press LLC, New
York, pp.53
Giraud-GuilleM M and Bouligand Y 1986
Chitin-protein molecular organization in
arthropods. In Chitin in Nature and Technology (ed. R.
Muzzarelli, C. Jeuniaux and G. W.
Gooday), pp. 29-35. New York: Plenum Press.
Greg, Lutz C and Jimmy L A 1999
Bullfrog Culture,
Southern Regional Aquaculture Center
Through Grant No, 94-38500-0045 from the Unites States
Department of Agriculture,
Cooperative States Research, Education and Extension Service, SRAC Publication No, 436.
http://govdocs.aquake.org/cgi/reprint/2003/724/7240150.pdf
Halter S Biodigester Development in
Saskatchewan
www.csale.usask.ca/PDF Documents/biodigester
Develop.pdf)
Hale O M 1973 Dried Hermetia illucens
larvae (Stratiomyidae) as a feed additive for poultry.
J.
Ga.Entomol. Soc. 8: 16-20.
KenchingtonW 1976 Adaption of insect peritrophic
membranes to form cocoon fabrics. In The
Insect Integument (ed. H. R. Hepburn), pp. 497-513.
Amsterdam: Elsevier Science.
KamonphoneT1977
Experiment Frog culture in Annual
Report 1966, Department of Fisheries,
Bangkok50-81 p.
Kramer K J and Koga D 1986 Insect chitin: physical state,
synthesis, degradation and metabolic
regulation. Insect Biochem. 16, 851-877.
Kramer K J, Hopkins T L and Schaefer J 1995 Applications
of solids NMR to the analysis of
insect sclerotized structures. Insect Biochem. Mol.
Biol. 25, 1067-1080.
LengRA, Stambolie JH and Bell RE 1994 Duckweed a
potential high protein feed resource for
Domestic animals and fish In: Improving animal production
systems based on local feed
resources. 7th AAAP Animal Science Congress
100-117.
LehaneM J 1997 Peritrophic matrix structure and
function. Annu. Rev.Entomol. 42,
525-550.
LARReC 2001 Memorandum and frog survey in Vientiane
prefecture, Living Aquatic Resources
ResearchCenter, dated 9/4/02.
LiengK, Phanousith S and Vongvichith B 2003Frog
Breeding and Nursing from Frog nursing
in Cage by used different feed, faculty Agriculture, National
University of Laos. Page. 32.
MarttinezI P, Herrez M P and Alvalez R 1993
Optimum Level of Dietary Protein For
Rana
pereziSeane larvae. Aquaculture and Fisheries
Management.271-278 p.
Men B X, Ogle B and T R Preston 2005
Production and
evaluation of Black Soldier fly larvae
and Termites as protein supplements for chickens http://www.mekarn.org/proctu/men25.htm
NewtonGL, CV Booram, RW Barker and OM Hale 1977
Dried Hermetiaillucens larvae
meal
As a supplement for swine. J. Anim. Sci., 44,
395-400
Nguyen Quang Suc, Le Thi Ha and Dinh Van Binh 2000 Manure
from rabbits, goats, cattle
And Buffaloes as substrate for earthworm. Workshop- seminar
"Making better use of local feed
resources" (Editor: T R Preston, B Ogle and Luu Trong Hieu)
SAREC-UAF, January, 2000, Ho
Chi Minh city, Vietnam http://www.mekarn.org/sarpro/sucew.htm
Peters W, Heitmann S and Haese J 1979 Formation and fine
structure of peritrophic
membranes in the earwig, Forficula auricularia.
Entomol. Gen. 5, 241-254.
Peters W 1992 Peritrophic membranes. In
Zoophysiology, vol. 30 (ed. S. D. Bradshaw,
W.
Burggren, H. C. Heller, S. Ishii, H. Langer, G. Neuweiler and D.
J. Randall), pp. 1-238. Berlin:
Springer.
ParlevlietG J 1997 Aqriculture westem Australiq
Senior Department officer south perth.6.P
(English).
PrestonTR and Rodríguez L 2002 Low-cost
biodigesters as the epicenter of ecological farming
systems; International Workshop on Research and Development on
Use of Biodigesters in SE
Asiaregion. Eds: Preston T.R., Nguyen Duong Khang. Ho Chi Minh
City, Vietnam, 10-11 March
2002. http://www.mekarn.org/procbiod/pres.htm
Rodriguez l and Preston T R No date Productive use of
livestock wastes; a manual for the use
of biodigester effluent and ponds for duckweed
production....
www.fao.org/WAICENT/FAOINFO/AGRICULT/AGA/AGAP/FRG/Recycle/dweed/mandw.htm
RudallK M and Kenchington W 1973 The chitin
system. Biol. Rev.48, 597-636.
Sheppard C No date Black Soldier Fly and Others for
Value-Added Manure Management.
http://www.virtualcentre.org/en/enl/vol1n2/article/ibs_conf.pdf
SpinelliNo dateAquaculture development and
coordination programme, Fish feed technology.
http://www.fao.org/docrep/X5738E/x5738e0d.htm
SamphoneK and P Bounsuang 1999 Culturing
earthworms on training course in Vietiane city,
Laos. Page. 7.
ThongyountT 2004Reported Frog Processing and Frog
Culture, Faculty of Fisheries
Latsmongkhon Technology Institute. 10-35 p. Natural and Semi
artificial, Living Aquatic
Resource Research Center, National Agriculture and forestry
Research Institute, Ministry of
Agriculture and forestry. 15-30 p.
Tram N D Q 2005
Culturing earthworms on pig manure and
the effect of replacing trash fish by
earthworms on the growth performance of catfish (MEKARN MSc
2003-2005.
Uodone2004 Frog hatchery on training course in
Vietiane city, Laos. Page. 12.
VermeulenC A and Wessels J G 1986 Chitin
biosynthesis by a fungal membrane preparation.
Evidence for a transient non-crystalline state of chitin.
Eur. J. Biochem. 158, 411-415.
Vines A E ND N Rees 1997 Plant and Animal biology volume
L, Department, the college of st.
Mark and st. John, cheisea, PP 6-603 (Enhlish).
Viet C 2001 Preduction of larvae and earthworms to feed
farm animal. Ho Chi Minh Publishing House. pp. 47-68