Ensiled taro (Colocasia esculenta) leaves in diets of local crossbred
pigs in Cambodia
Pheng Buntha
Center for Livestock and Agriculture
Development, Phnom Penh, Cambodia
buntha@celagrid.org
Literature Review
Livestock production systems in
Cambodia
In a baseline survey conducted by CelAgrid (2006a), pig keeping was found not to be very attractive due to the high cost of feeding. The fluctuations in price per unit live weight were another reason that discouraged farmers from keeping pigs as a source of income.
Within the livestock sub-sector however, pig production has
shown a significant increase during the last 4 years (Table 1). The
increase in production is mainly due to the growth of medium-scale
farms around cities to satisfy the demand for meat of the
increasing urban population.
Pig production systems in Cambodia can be classified into traditional, semi-intensive and intensive production (Maclean 1998).
|
Table 1. Livestock population in Cambodia (2000-2004) |
||||||
|
|
2000 |
2001 |
2002 |
2003 |
2004 |
% increase |
|
Cattle |
2,992,640 |
2,868,827 |
2,924,457 |
2,985,416 |
30,354,002 |
9.14 |
|
Buffaloes |
693,631 |
626,016 |
625,912 |
660,493 |
680,500 |
-0.02 |
|
Pigs |
1,933,930 |
2,114,524 |
2,105,435 |
2,304,248 |
24,842,501 |
11.9 |
|
Poultry |
15,249,201 |
15,248,447 |
16,677,864 |
16,013,713 |
16,033,700 |
0.05 |
|
Source: MAFF, 2004 |
||||||
Traditional pigs feeding and management
systems
The tradition average for pig ownership is about 1.6/rural household, with large variations between households. Given the high capital cost and management expertise required, pig breeding tends to be carried out by the better-off farmers, whereas raising a single small pig purchased from the breeder is the typical activity of poor farmers, from whom it is a way of capital accumulation, with a small initial investment, although still rather risky given the absence of health services (Maclean 1998).
FAO (2005) reported that the definition of a small-holder pig farm varies amongst countries. For instance, in Philippines and Vietnam a small farm has less than 20 pigs, while small farms in Cambodia and Laos have less than 5 pigs. Smallholder farmers are mainly located in rural areas, and because of their numbers and generally low standard of living, smallholders are an important focus for poverty alleviation and development programs sponsored by the Government and donor agencies (Sen Sovann 2002).
Semi-intensive and intensive pig production
systems
At the moment, there are only a few commercial pig farms in Cambodia, mainly located near Phnom Penh (eg.Yu Tong and CP companies). They supply almost all the grandparent stock, breeding sows and piglets to medium-scale producers, particularly around Phnom Penh and other cities. These farms are very well-equipped, well-managed and have a high productivity. Only exotic breeds are kept in this system, mostly Yorkshire, Landrace and Duroc. Around Phnom Penh and other cities in Cambodia, a medium sized pig farm may have 10-50 pigs, with a mix of production categories such as sows, piglets and fatteners (Khieu Borin personal communication).
Distribution of taro (Colocasia esculenta) in the
World
The Araceae is a large family, comprising some hundred genera and more than fifteen-hundred species. Mostly tropical or subtropical plants, the aroids grow mainly in moist or shady habitats. Some are terrestrial plants while others are vines, creepers, or climbers. Many species of the Araceae are also epiphytes. The major edible aroids are classified in two tribes and five genera; Lasioideae (Cyrtosperma and Amorphophallus) and Colocasiodeae (Alocasia, Colocasia, and Xanthosoma). Taro, Colocasia esculenta (L.) Schott is considered as a single polymorphic species (Lee 1999).
The Taro plant is a perennial herb with clusters of long heart- or arrowhead-shaped leaves that point earthward. Taro leaves grow on erect stems that may be green, red (lehua), black or variegated. The new leaves and stems push out of the innermost stalk, unrolling as they emerge. The stems are usually several feet high. Taro bears a short underground stem called a corm, where the plant stores starch produced by the leaves. In the eight to sixteen months of its development, the corm can grow as large as six inches in diameter. People raise taro to obtain this valuable starchy root. When the plant reaches maturity, it will produce a flower stalk in some leaf axils. Near the apex of the flower stalk appears the yellow-white, tubular spathe, or modified leaf, which covers and protects the flower cluster within. Inside grows an erect spike called the spadix. The spadix bears two kinds of flowers: the male and the female flowers. The male flowers lie toward the upper part of the spadix, and the female flowers lie toward the lower part. Tiny new plants appear around the base of the root corm (Lucas 1982).
In Ancient Hawaii, before 1778, about 300 named varieties of Taro were grown. Subsequently, Hawaiians cultivated Taro inter-cropped with other species, such as erythrina, banana, papaya, coconut and green peas, which ensures the maintenance of the health of Taro species. The 20th century brought the monoculture technique, which made Taro very much more susceptible to diseases. Now, in the 21st century there are only 7 to 12 varieties cultivated in Hawaii. Diseases, such as fungal disease and pocket rot, and the introduced apple snails have severely impacted Taro cultivation in Hawaii (Tavana 2003).
In Cambodia, there are several varieties of taro found in the rural areas. However, two main varieties are planted, according to the soil conditions, and wild taro plants also grow naturally in the forests, ponds, canals etc. and are harvested (Paper I).
Ecology
In the early days, there were more than 300 named varieties of Taro. Approximately 87 of these varieties are still recognized today, with slight differences in height, stalk color, leaf or flower color, size, and corm type (Lucas 1982).
The generic name "Colocasia" is a latinization of the Greek word "kolokasion". This name has been used, according to the first-century Greek physician and herbalist Dioscorides, for the root of the lotus, Nelumbo nucifera, and borrowed for used as the scientific name for Taro. "Esculenta" means esculent or edible. This species is thought to be a native of India and perhaps other parts of southern Asia and from there its cultivation has extended. It reached Egypt about 2,000 years ago and spread into the Pacific area in ancient times (Green 1996).
Anti-nutritional factors
Before Taro can be eaten, all parts of the plant must be cooked, in order to break down the needle-like calcium oxalate crystals present in the leaves, stem and corm. These crystals can be extremely irritating to the throat and mouth lining, causing a burning and stinging sensation (Miller 1929). Taro contains substantial quantities of oxalate (Standal 1983), as do the majority of higher plants (82 of 93 orders) (Zindler-Frank 1976). The ubiquity of oxalate in plants has raised questions about its roles. Proposed functions of oxalate include protection from insects and foraging animals through toxicity and/or unpalatability, osmoregulation, and regulation of Ca levels in plant organs and tissues (Libert and Franceschi 1987).Cellular oxalate, widely distributed in many plants, is implicated inplaying important roles in various functions and is also known to affect food qualities adversely in fruits and vegetables. How oxalate is regulated in plants is currently not well understood. Glycolate oxidase (GLO) has long been considered as an important player in oxalate accumulation in plants. To gain further insight into the biochemical and molecular mechanisms, the possible roles of GLO in the process were studied. Drastically different levels of oxalate could be achieved by treating rice with various nitrogen forms (nitrate versus ammonium). While nitrate stimulated oxalate accumulation, ammonium reduced its level. Such treatments resulted in similar pattern changes for some other related organic acids, such as glycolate, oxaloacetate, and malate (Hua-Wei et al 2006).
Noonan et al (1999) reported that oxalic acid and its salts occur as end products of metabolism in a number of plant tissues. When these plants are eaten they may have an adverse effect because oxalates bind calcium and other minerals. While oxalic acid is a normal end product of mammalian metabolism, the consumption of additional oxalic acid may cause stone formation in the urinary tract when the acid is excreted in the urine. Soaking and cooking of foodstuffs high in oxalate will reduce the oxalate content by leaching. The high content of calcium oxalate crystals (about 780 mg per 100 g in some species of cocoyam, Colocasia and Xanthosoma) has been implicated in the acridity or irritation caused by cocoyam. Oxalate also tends to precipitate calcium and makes it unavailable for use by the body. These crystals can be extremely irritating to the throat and mouth lining, causing a burning and stinging sensation (Miller 1929).
Oke (1967) has given an extensive review of the role of oxalate
in nutrition, including the possibility of oxalaurea and kidney
stones. The acridity of high oxalate cultivars of cocoyam can be
reduced by peeling, grating, soaking and fermenting during
processing. In Cambodia, fresh wild taro leaves contain around 3.0
% of calcium oxalate, that causes severe itching.
Nutritive value of taro
Taro leaves are rich in nutrients, including minerals such as calcium, phosphorus and iron, and vitamin C, thiamine, riboflavin and niacin (FAO 1993; Barush 2002) (Table 2). The roots are rich in a starch composed of amylose (28%) and amylopectin (72%). Taro contains thiamine (vitamin B1), riboflavin (vitamin B2), niacin, oxalic acid, calcium oxalate and a sapotoxin. The tubers contain amino acids and high molecular weight proteins which inhibit the porcine pancreatic amylases. The corms contain the anthocyanins pelargonidin 3-glucoside, cyaniding 3-rhamnoside, and cyaniding 3-glucoside. Hydroxycinnamoyl amides have been obtained from the inflorescences and two new dihydroxysterols have been isolated from the tubers (Cambie and Ash 1994).
|
Table 2. Chemical composition of leaves, petiole and tuber of taro (Colocasia esculenta), % in DM |
|||||
|
|
Taro leavesa |
Taro leavesb |
Taro petiolea |
Taro tubera |
Taro tuberb |
|
DM |
- |
8.2 |
- |
- |
26.2 |
|
CP |
24.3 |
25 |
8.00 |
10.4 |
8.7 |
|
CF |
- |
12.2 |
- |
- |
1.7 |
|
EE |
4.07 |
10.7 |
1.80 |
0.17 |
0.4 |
|
Ash |
17.3 |
12.4 |
27.4 |
6.29 |
4.0 |
|
NFE |
- |
39.8 |
- |
- |
85.2 |
|
Ca |
1.44 |
1.74 |
0.67 |
0.15 |
- |
|
P |
0.13 |
0.58 |
0.13 |
0.10 |
- |
|
Oxalic acid |
5.97 |
- |
1.29 |
0 |
- |
|
Sources: a Baruah 2002, bFAO 1993 |
|||||
Methods for reducing the oxalic acid and improving
the quality of Taro leaves
When a crop is being considered for food, nutritional value and consumer acceptance must be taken into consideration. The nutritional value of a food depends upon its nutritional contents and their digestibility and the presence or absence of antinutrients and toxic factors (Lee 1999). However we can improve the quality or reduce the antinutritionals in crops by several ways, such as ensiling, chopping, wilting, drying, washing, boiling or using chemicals.
Tiep et al (2005) carried out a study on the processes for reducing the oxalic acid content, and use of Alocasia macrorrhiza roots for pig feed. The results showed that calcium oxalate could be reduced by chopping, to disrupt the cellular structure of the root, and then soaking the roots in NaOH. After processing the root meal could be included at levels of up to 50% in diets for growing pigs. Agwunobi et al (2002) evaluated five diets that comprised 0, 50% and 100% un-boiled, sun-dried taro cocoyam cormels (Colocasia esculenta) and 50% and 100% boiled, sun-dried taro cocoyam cormels as replacement for maize. The levels of some antinutritional factors were also determined in both boiled and un-boiled, sun-dried taro cocoyam. Boiling reduced the amounts of the antinutritional factors in the taro cocoyam cormels.
Tiep et al (2006) carried out a study where Alocasia macrorrhiza leaves (of White Alocasia or Khoai Ray), including the stems, were chopped into small species 2 - 3 cm in length, sun-dried for 1 -2 hours, and then mixed with 5, 7 or10 % rice bran and 2% molasses (on DM basis). The ingredients were mixed thoroughly and kept in plastic bags, tied tightly to keep anaerobic conditions and stored at room temperature (25 - 35oC) and the calcium oxalate content was reduced markedly by ensiling.
Ensiling
Ensiling is a process of fermentation of carbohydrates by acidification, and is a suitable method for feeds that are seasonally abundant for later feeding during periods of feed shortage (McDonald et al 2002). Almost any crop can be preserved as silage, but the commonest are grasses, legumes and whole cereals, especially wheat and maize. There are two main objectives in preserved crops. The first essential objective in preserving crops by natural fermentation is the achievement of anaerobic conditions. In practice this is done by chopping the crop during harvesting, by rapid filling of the silo and by adequate consolidation and sealing. The second essential objective is to discourage the activity of undesirable microorganisms such as clostridia and enterobacteria, which produce objectionable fermentation products (McDonald et al 2002). However, Ohio Sate University Extension (2001) said that the main function of a silage additive is to increase the nutritional value or improve fermentation. Holmes and Brookes (2001) reported that high quality silage is a stable feed, made from high quality pasture, which is preserved in the absence of oxygen by a high quality fermentation, to minimise any loss of feeding value. It is impossible to produce high quality silage from low quality pasture, no matter how good the fermentation is. Both the quality of the ensiled pasture and the quality of the fermentation must be considered. These can be measured by having a feed analysis done on the silage.
Role of plant enzymes in ensilage
Immediately after cutting the crop and during the early stages of ensiling, chemical changes occur as a result of the activity of enzymes present in the plant tissue. The processes of respiration and proteolysis are of particular importance in influencing the nutritional value of the final product (McDonald et al 2002).
Role of microorganisms in ensilage
The microorganisms that grow most rapidly will be predominantly lactobacilli species, which produce lactic acid that will lower the pH of the silage. Fermentation completely ceases after 3 or 4 weeks when the pH becomes so low (<4.5) that all the microbial growth is inhibited (Bjorge 1996). Aerobic fungi and bacteria are the dominant microorganisms on the fresh herbage, but as anaerobic conditions develop in the silo they are replaced by bacteria able grow in the absence of oxygen. This includes lactic acid bacteria, clostridia and enterobacteria (McDonald et al 2002).
Nutrient losses in ensilage
During crop harvesting and ensilage there can be a loss of nutrients in the field, and due to oxidation, fermentation, and in effluent. In the field, nutrient losses are negligible and even over a 24-hour wilting period, losses of dry matter of not more than 1 or 2 percent may be expected. Dry matter losses are around 6 percent after wilting five days and 10 percent after eight days. Oxidation losses are a result of the action of plant and microbial enzymes on substrates, such as sugars, in the presence of oxygen, with the concomitant formation of carbon dioxide and water. The oxygen trapped within the plant tissues is of little significance and causes dry matter losses of about 1 percent only. Biochemical changes occur during fermentation, especially in the soluble carbohydrates and protein, but overall dry matter and energy losses arising from the activities of lactic acid bacteria are low. Dry matter losses can be expected to be less than 5 percent and gross energy losses, because of the formation of high-energy compounds such as ethanol, are even less. In clostridial and enterobacterial fermentation, nutrient losses will be much higher than in lactic acid bacterial fermentations because of the evolution of the gases carbon dioxide, hydrogen and ammonia. In effluent losses, the free drainage occurs and the liquid, or effluent, carries with it soluble nutrients. The amount of effluent produced depends largely upon the initial moisture content of the crop. Several equations, based on the dry matter of the ensiled material, have been suggested for estimating effluent loss. As well as dry matter, factors such as type of silo, degree of consolidation and the nature and pretreatment of the crop will affect effluent loss, but it will obviously be increased if the silo is left uncovered so that rain enters (McDonald et al 2002).
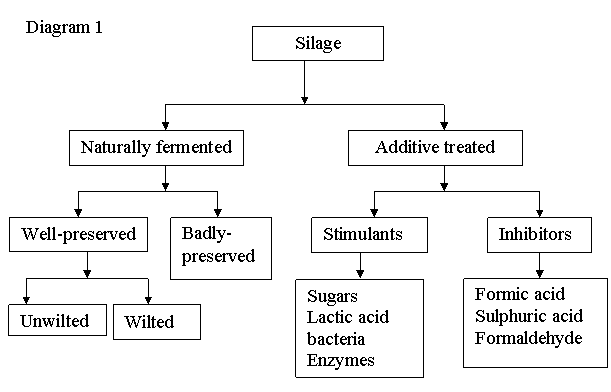
Classification of silage
Silage may be classified into two main categories. In the first, which is naturally fermented, commonly made from grasses and whole cereal crops, lactic acid bacteria dominate the fermentation. A typical pattern is show in Diagram 1. This silage is characterized by having low pH values, usually between 3.7 and 4.2 and containing high concentrations of lactic acid. The second, additive-treated, can be classified into two main types: with fermentation stimulates, such as sugar-rich material, inoculants and enzymes, which encourage the development of the lactic acid bacteria; and with fermentation inhibitors such as acids and formalin, which partially or completely inhibit microbial growth (McDonald et al 2002).
Ensiling Taro leaves
Forage ensiling is based on storage of the forage under anaerobic conditions that inhibits the activities of undesirable microorganisms. A pH of about 4.0 will normally preserve the crop satisfactorily. Wet crops are very difficult to ensile satisfactorily unless they are either pre-wilted under good weather conditions or treated with a suitable additive. Similarly, forages low in water-soluble carbohydrates, or those which are highly buffered must also be treated with an effective additive before ensiling. Legumes are known to be more difficult to ensile, because they have a high buffering capacity, a low content of water soluble carbohydrates, and often a low DM content (Van Soest 1994; McDonald et al 2002). However, Taro (Colocasia esculenta) is considered to be a wet crop due to the low DM content of leaves (20.8% of DM) and is ensiled to reduce the oxalic acid content. It is not difficult to ensile because the pH decreased quickly from 6.14 to 4.60 after one week and fell to 4.2 after 4 weeks when 100 kg of fresh Taro leaves was mixed with 5% of sugar palm syrup (80% of DM).
Chittavong et al (2006) carried out a study where taro leaves were chopped into small pieces (2 to 3 cm) and ensiled in plastic bags (capacity 2 kg) with levels of sugar cane molasses of 0, 2, 4 and 6% (DM basis). After 7 days the colour for all treatments had changed from green to yellow-brown and was darker at higher levels of molasses. Each treatment had an acceptable smell. The pH values for all treatments were around 6 at day 0 and then quickly fell below 5, the value being dependent on ensiling time and the level of molasses (P<0.05). At day 0 the concentration of NH3-N was very low on all treatments, but from 7 days onwards the concentration had increased with the time of ensiling on all treatments. The ammonia-N concentration decreased as the level of molasses increased. A level of 4% molasses and an ensiling period of between 14 and 21 days appeared to be the most appropriate for ensiling Taro leaves, as determined by pH, ammonia concentration and water extractable DM and N.
However taro leaves can be ensiled with other material such as rice bran or salt. Tiep et al (2006) ensiled giant taro (Alocasia macrorrhiza) leaves with three levels of rice bran (5, 7 and 10 %) or with 2% molasses (DM basis) as additives. Ensiling the Alocasia macrorrhiza leaves with 7% rice bran and 2% molasses reduced the calcium oxalate content by 78.8%, maintained the pH at 4.05-4.12 until the 60th day, and improved palatability, without reducing the nutritive value of the silage.
Energy feeds for crossbred growing
pigs
For the farmers the essential steps in the scientific rationing of their animals are, first, the assessment of their nutrient requirements, and second, the selection of foods which can supply these requirements. This balancing of demand and supply is made separately for each nutrient, and in many cases the nutrients given first consideration are those supplying energy. These are good reasons why energy should receive priority. In the first place the energy supplying nutrients are those present in the food in the greatest quantity; this means that if the diet has been devised to meet other nutrient requirements first and is then found to be deficient in energy, a major revision of its constituents will probably be needed. In contrast, a deficiency of a mineral or vitamin can often be rectified very simply by adding a small quantity of a concentrated source.
An animal deprived of food continues to require energy for those functions of the body immediately necessary for life - for the mechanical work of essential muscular activity, for chemical work such as the movement of dissolved substances against concentration gradients, and for the synthesis for expended body constituents such as enzymes and hormones. In the starving animal the energy required these purposes is obtained by the catabolism of the body's reserves, first of glycogen, then of fat and protein. In the fed animal the primary demand on the energy of the food is in meeting this requirement for body maintenance and so preventing the catabolism of the animal's tissues (McDonald et al 2002). There are many locally available resources that can provide energy for pigs in Cambodia.
Sugar palm syrup is made from sugar palm juice by boiling to convert liquid to solid. The sugar palm juice can be processed into 3 types of sugar at small farm level: liquid sugar (sugar palm syrup), solid palm sugar and block sugar. The most common type for the consumption in the countryside is sugar palm syrup, which has about 80% dry matter content. In addition sugar palm juice has a high nutritive value due to it high soluble carbohydrate content (98.4% of dry matter) (Paulas and Muthukrishnan 1983). Khieu Borin (1996) found that the average Brix value of palm juice was 13.3%. The composition of the palm syrup shows considerable variation among farmers and periods. Sucrose as a percent of total solid in juice ranged from 66 to 94% in samples taken in January and from 51 to 88% in April. In contrast, the glucose and fructose levels in juice increased. The levels of glucose ranged from 2.1 to 9.6% in samples taken in January and from 3.5 to 18.2% in April. The fructose levels ranged from 2.6 to 11% in samples taken in January and from 4.6 to 24.5% in April. Feeding pigs with the palm juice or sugar cane juice as the main dietary energy source was shown to be an alternative for sustainable production (Fernandez 1988; Mena 1987; Elliot and Kloren 1987; Preston 1991; Beech et al 1990; Speedy et al 1991; Phuc et al 1994a and b; Khieu Borin 1996).
Maize, like the other cereal grains, has certain limitations as a food for farm animal. Though an excellent source of digestible energy it is low in protein, and the proteins present are of poor quality. Maize contains about 730g starch/kg DM, is very low in fiber and has a high metabolisable energy value. The starch in maize is more slowly digested in the rumen than that of other grains, and at high levels of feeding a proportion of starch passes into the small intestine, where it is digested and absorbed as glucose. The oil content in maize varies from 40 to 60 g/kg DM and is high in linoleic acid, which is an important factor in that it tends to produce a soft body fat (McDonald et al 2002)
Rice bran is commonly used for pigs by rural farmers in Cambodia, because it is the residue from grinding rice and is cheap and available in the villages. In fact, the raising of pigs may be viewed partly as a means of adding value to rice bran or just to save income when rice bran is purchased. Rice bran, being of medium energy and protein content, is in itself a reasonable feed for pigs, and it has been estimated that rice bran accounts for up to 50% of the feed for pigs in Cambodia. Farmers buy the rice bran from the rice miller, who owns the bran as part payment for the milling process. The majority of rice grown in Cambodia is processed in local "village" mills. The miller usually takes the by-products of milling - cracked and fine cracked grain, bran and husk as payment for the service of milling. The rice bran available from village mills is generally poor quality due the high content of husk. The rice bran from larger mills with modern machinery is of better quality, with 12-13% crude protein, while in bran from village mills the protein content is only 8-10%. In the market, different qualities of bran can be bought, generally classified as first, second and third grade. Third grade has significant amounts of husk. Husk has a high content of fibre, which pigs can not digest, therefore brans with high levels of husk are poorer quality feeds.
Protein supplements
It is important to provide feed to pigs with the necessary amount of protein containing a well balanced profile of essential amino acids. It is known that if any one essential amino acid is lacking, the others can not be efficiently utilized by the pig, and also excess amino acids are deaminated in the liver. The nitrogen is excreted in the urine and the remaining deaminated fraction is used as an energy source. Therefore, it is not recommendable to provide this expensive nutrient in a higher quantity than needed (Holness 1993).
Conventional protein resources
In 1999, the world production of fishmeal was 6 247 000 tonnes. Fishmeal is produced by cooking fish, and pressing the cooked mass to remove most of the oil and water. The aqueous liquor is concentrated added to the pressed mass and the whole dried. About 90 per cent of the raw material used in fishmeal production consist of oily species such as anchovies, capelin and menhaden, the remaining 10 per cent consisting of fish (plus some of the offal) of species such as haddock and cod. Nutritionally, the drying process is very important since overdrying may significantly reduce the quality of the product.
In well-processed meals the protein has a digestibility of between 0.93 and 0.95, but meals heated too strongly in the processing may have values as low as 0.60. The quality of protein in fishmeal is high though variable, as indicated by values of 0.36 and 0.82 quoted as the biological value for rats.
The protein contents of various fish meals vary over a range of about 500-750 g/kg but the composition of the protein is relatively constant. It is rich in essential amino acids, particularly lysine, cystine, methionine and tryptophan, and is a valuable supplement to cereal-based diets, particularly where they contain much maize. The essential amino acid composition of fish meal is compared with that of ideal protein in Table 3.
|
Table 3. Ratio of the essential amino acids to lysine in fish meal protein and Ideal Protein |
||
|
Amino acid |
Fish meal |
Ideal protein |
|
Lysine |
1.0 |
1.0 |
|
Methionine + cystine |
0.45 |
0.50 |
|
Tryptophan |
0.14 |
0.14 |
|
Threonine |
0.57 |
0.60 |
|
Leucine |
1.01 |
1.00 |
|
Isoleucine |
0.62 |
0.54 |
|
Valine |
0.73 |
0.70 |
|
Histidine |
0.29 |
0.33 |
|
Phenylalanine + tyrosine |
1.04 |
0.96 |
|
Source: McDonald et al., 2002 |
||
Fish meals have a high mineral content (100-220 g/kg), which is of value nutritionally since it contains a high proportion of calcium and phosphorus and a number of desirable trace minerals including manganese, iron and iodine. They are also a good source of B complex vitamins, particularly choline, B12 and riboflavin, and have enhanced nutritional value because of their content of growth factors known collectively as the animal protein factor (APF).
The energy of fish meals is present entirely in the form of fat and protein and is largely a reflection of the oil content. In the past, the energy value for animals has been underestimated, particularly in the case of ruminants, for which average values in the region of 14 MJ/kg of metabolisable energy are now accepted as realistic.
A wide variety of fish meals are available depending upon the country of origin, the raw material and the process used. The current trend in the marketing of fish meals is towards specialised products tailored to suit particular species. Fish meals find their greatest use with simple-stomached animals. They are used mostly in diets for young animals, whose demand for protein and the indispensable amino acids is particularly high and for whom the growth-promoting effects of APF are available. Such diets may include up to 150 kg/tonne of fish meal. With older animals, which need less protein, the level of fish meal in the diet is brought down to about 50 kg/tonne and it may be eliminated entirely from diet for those in the last stages of fattening. The composition and nutritive value are given in Table 4 (McDonald et al 2002).
|
Table 4. Composition and nutritive value of some typical fish meals |
||||
|
|
Herring |
South American |
UK produced |
This study |
|
Crude protein, g/kg |
730 |
660 |
640 |
356 |
|
Oil, g/kg |
70 |
60 |
65 |
- |
|
Ash, g/kg |
- |
- |
- |
568 |
|
Calcium, g/kg |
20 |
45 |
80 |
- |
|
Phosphorus, g/kg |
15 |
30 |
50 |
- |
|
ME, MJ/kg DM |
||||
|
Ruminants |
17.8 |
14.6 |
14.6 |
- |
|
Poultry |
14.9 |
13.9 |
13.4 |
- |
|
DE, MJ/kg DM (pigs) |
19.6 |
19.0 |
17.0 |
- |
Soya bean meal contains from 160 to 210 g/kg of oil and is normally solvent extracted. The meal is generally regarded as one of the best sources of protein available to animals. The protein contains all the essential amino acids but the concentration of cystine and methionine are suboptimal. Methionine is the first limiting amino acid and may be particularly important in high-energy diets.
Soya bean meal contains a number of toxic, stimulatory and inhibitory substances including allergenic and anticoagulant factors. Of particular importance in nutrition are the protease inhibitors, of which six have been identified. Two of these, the Kunitz anti-trypsin factor and the Bowman-Birk chymotrypsin inhibitor, are of practical significance. The protease inhibitors are partly responsible for the growth-retarding property of raw soya beans or unheated soya bean meal. The retardation has been attributed to inhibition of protein digestion but there is evidence that pancreatic hyperactivity results in increased production of trypsin and chymotrypsin. The consequent loss of cystine and methionine accentuates the marginal status of soya bean meal with regard to these acids and results in induced deficiencies of both. The Kunitz factor, but not the Bowman-Birk, is inactivated (30-40 per cent) by human gastric juice in vivo at pH 1.5-2.0. The Bowman-Birk factor is inactivated in its passage through the intestine of the chick. About half the growth-retarding effect of soya bean meal in monogastric animals has been attributed to the lectin content. Their toxicity varies, being extreme in the castor bean but relatively mild in the soya bean. The inhibitors are inactivated by heating, which accounts for the preference shown for toasted meals for simple-stomached animals. For ruminant animals, the inhibitors are not important and toasting is unnecessary. The process of toasting must be carefully controlled since overheating will reduce the availability of lysine and arginine and reduce the value of the protein. As long as adequate supplementation is practiced it may be included at up to 250 kg/tonne in pig diets (McDonald et al 2002).
Alternative sources of protein
A cheap alternative source of protein could be green leaves and water plants. The green part of biomass is potentially the most abundant protein source (D'Mello 1995). Due to their high potential of protein production per hectare, and as they are not directly used in human nutrition, these plant species could be a "non-competitive" protein source for monogastric animal species. However the major problems, which preclude the used of these plants as protein sources for monogastrics, are their limited palatability and high levels of fibre, which may limit the feed intake and availability of nutrients (Cheeke et al 1980; Rosales et al 1993). Water plants and leaves (Table 5), such as duckweed, Azolla, water hyacinth, sweet potato leaves, cassava leaves, sweet potato vines, Indicago leaves, groundnut foliages, leucaena leaves, mung bean foliages and trichantera leaves can be used by small scale pig producers in Cambodia.
|
Table 5. Chemical composition of some unconventional protein supplements |
||||||
|
|
----As % of dry matter ---- |
|||||
|
|
DM |
CP |
CF |
Ca |
P |
Reference |
|
Water spinach |
9.6 |
27.1 |
16.4 |
1.1 |
0.5 |
Naren et al 1994 |
|
Duckweed |
4.7 |
38.6 |
18.7 |
0.7 |
0.6 |
Men et al 1995 |
|
Azolla |
5.6 |
26.7 |
8.4 |
0.4 |
0.5 |
Becerra et al 1995 |
|
Water hyacinth |
7.8 |
12.8 |
24.6 |
2.1 |
0.4 |
Gohl 1994 |
|
Sweet potato vines |
14.2 |
18.5 |
23.5 |
- |
- |
Domínguez 1992 |
|
Sweet potato leaves |
8.7 |
21.9 |
15.0 |
1.8 |
0.2 |
Gohl 1994 |
|
Leucaena leaves |
25.5 |
28.3-30.2 |
15.7 |
- |
- |
Phuc 2000 |
|
Indicago leaves |
- |
26.2 |
25.5 |
- |
- |
Gohl 1981 |
|
Groundnut foliages |
26.9 |
17.5 |
20.1 |
- |
- |
Gohl 1981 |
|
Mung bean foliages |
16.0 |
19.4 |
26.8 |
- |
- |
Gohl 1981 |
|
Trichantera leaves |
20.0-26.0 |
15.1-22.5 |
16.7-18.3 |
- |
- |
Rosales 1997 |
|
Cassava leaves |
16.1 |
24.1 |
26.0 |
1.0 |
0.5 |
Gohl 1994 |
Feed digestibility in the pig
In the adult pig, the digestive tract can be considered as a tube extending from mouth to anus, lined with mucous membranes, whose function is the prehension, ingestion, comminution, digestion and absorption of food, and elimination of solid waste material.
Digestion in the mouth is mainly mechanical, mastication helping to break up large particles of food and to mix them with saliva, which acts as a lubricant and is a medium for taste perception. The pig has taste buds throughout the oral cavity and they are concentrated on the tongue.
The stomach of the adult pig has a capacity of about 8 litres and consists of a simple compartment, which functions not only as an organ for digestion of food but also for storage. The α-amylase activity may continue and there is an active microbial population, mainly lactobacilli and streptococci. Thus the gastric juice consists of water, pepsinogens, inorganic salts, mucus, hydrochloric acid and the intrinsic factor important for the efficient absorption of vitamin B12 that are secreted into the stomach and mixed together with food during digestion.
The partially digested food leaving the stomach enters the small intestine, where it is mixed with secretions from the duodenum, liver and pancreas. The majority of digestion and absorption occurs in the small intestine, the duodenal area being the site for mixing digesta and secretions and jejunal area being the site of absorption.
The main site of absorption of digested nutrients is the small
intestine; by the time the food material has reached the entrance
to the colon most of the hydrolysed nutrients have been absorbed.
With normal diets there is always a certain amount of material
which is resistant to the action of the enzymes secreted into the
alimentary canal. The large intestine plays an important role in
the retrieval of nutrients, electrolytes and water in the digesta.
The waste material, or faeces, voided from the large intestine via
the anus consists of water, undigested food residues, digestive
secretions, epithelial cells from the tract, inorganic salts,
bacteria and products of microbial decomposition (McDonald et al
2002).
Conclusions
-
Taro is a considered to be good vegetable crop and is commonly planted for human consumption near the farms in the two different agro-ecological zones in Cambodia that were surveyed. There are many varieties of taro grown, but in this study it was found that there were two main varieties of taro cultivated, Chouk and Sla, and a wild variety (Trav Prey) is also available in the villages. Both the cultivated varieties are short-term, and can be harvested 5-8 months after planting. The main purpose is the root or tuber for human consumption. However the petiole is also boiled as a vegetable for human consumption, and petioles and leaves are sometimes boiled with rice bran or broken rice for pigs. The limiting factor in the use of taro for pigs is the itching factor, caused by the high oxalic acid content, and lack of knowledge or information on how to use it. The acridity of high oxalate cultivars of cocoyam can be reduced by peeling, grating, soaking, drying, boiling and fermenting during processing.
-
Ensiled taro leaves are a protein source of potential value for small pig producers in Cambodia. The protein is well balanced in essential amino acids but not well digested by pigs. Ensiled taro leaves can replace up to 70-75% of the protein from fish meal in diets based on sugar palm syrup. The limiting factor to achieving higher levels of substitution appears to be the low apparent digestibility of the protein in the taro leaves and its association with the fibrous fraction.
-
For improving pig production in the rural areas of Cambodia, it is important to consider the profitability of using locally available protein and energy resources. Taro leaf silage can be used for pig feeds, but more studies are required to evaluate other processing techniques and also the use of the petioles.
Acknowledgments
The studies were carried out during 2006 at the Center for
Livestock and Agriculture Development (CelAgrid-UTA Cambodia),
Kandal district, and in the districts of Phnom Krovagn and Trang,
in Pousat and Takeo provinces, respectively. The studies are part
of the requirement for the Master of Science degree in "Sustainable
Livestock Systems", awarded by the Department of Animal Nutrition
and Management, Swedish University of Agricultural Sciences, and
were made possible through the financial support of the Swedish
International Development Agency, Department for Research
Cooperation (Sida-SAREC). I also would like express my deep
gratitude to my main supervisors, Professor Brian Ogle and Dr T R
Preston, and my local supervisor, Dr.Khieu Borin, Director
of CelAgrid, for their support, valuable advice and useful guidance
during my studies and research project. I would also like to thank
Professor Inger Ledin, for her kind assistance during my course
work in Cambodia, Laos, Thailand and Vietnam, and all other
professors and lecturers for their great support and scientific
guidance during the courses. Finally I would like to thank the
farmers in the six survey villages and the PRA team who
participated in the survey, and also the students and staff of
CelAgrid who cooperated with and assisted me in carrying out this
research.
References
Agwunobi L N, Anwukam P O, Cora O O and Isika M A 2002.
Studies on the use of Colocasia esculenta (Taro cocoyam) in
the diets of weaned pigs. Trop. Anim. Health. Prod 34 (3):
241-247.
http://www.springerlink.com/content/wxknvypgpc97b419/?p
AFRIS 2004. Animal Feed Resources Information Systems.
Updated from B. Göhl, (1981) Tropical feeds. Food and
Agriculture Organization.http://www.fao.org./ag/AGa/agap/FRG/AFRIS/DATA/535.htm
Baruah K K 2002. Nutritional Status of livestock in
Assam. Agriculture in Assam. Pub Directorate of Extension, Assam
Agri. Univer. Pp : 203.
Becerra M, Ogle B and Preston T R 1995. Effect of
replacing whole boiled soya beans with azolla in the diets of
growing duck. Livestock Research for Rural Development, 7(3): pp
32-38.
http://www.cipav.org.co/lrrd/lrrd/7/3/8.htm
Beech S A, Elliott R and Betterham E S 1990.
Sucrose as an
energy source for growing pigs: Digestibility energy content and
energy utilization. Animal. Production. 51: 343-355.
Bjorge M 1996: Ensiling process. Alberta Agriculture, Food and
Rural Development.
Cambie R C and Ash J 1994. Fijian Medicinal
Plants. CSIRO, Australia pp365.
CelAgrid 2006
Baseline survey in Phnom Kravagn district,
Pursat province. Project supported by FSIF/CIDA.
Cheek P R, Telek L and Carlsson R 1980.Nutritional
evaluation of leaf protein concentrates prepared from selected
tropical plants. Nutr. Rep. Int. 22 (5): 717-895.
Chittavong M, Preston T R and Ogle B 2006. Ensiling
leaves of taro (Colocasia Esculenta) with sugar cane molasses.
Workshop-seminar "Forages for Pigs and Rabbits" MEKARN-CelAgrid,
Phnom Penh, Cambodia, 22-24 August, 2006. Article # 15. Retrieved
February 8, 107, from
http://www.mekarn.org/proprf/mala.htm
D'Mello J P F 1995.Leguminous leaf meals in non-ruminant
nutrition. In: Tropical legumes in animal nutrition. (Eds) D'Mello
J P F and Devendra P, Biddles, Guildford, London. pp
247-281.
Dominguez P L 1992. Feeding of sweet potato to
monogastrics. In: Root, tubers, plantains and bananas in animal
feeding. (Eds) Machin D, Nyvold S. FAO Anim.Prod. Health
Paper
95: 217-233.
Elliott R and Kloren W R L 1987. The use of sugar in diet
for monogastrics. Recent Advances in Anim. Nutr. in Australia. pp
290-296.
FAO 1993. Tropical Feeds by B. Göhl. Computerized
version 4.0 edited by A. Speedy, Rome, Italy. http://www.fao.org/docrep/003/w3647e/W3647E05.htm
FAO 2005. Livestock Sector Brief. Livestock Information,
Sector Analysis and Policy Branch, AGAL. pp 1-21.
http://www.fao.org/ag/againfo/resources/en/publications/sector_briefs/lsb_KHM.pdf.
Fernandez G M 1988. Uso de jugo de cana para cerdos en
una finca de mediano productores en la Republica Dominicana. In:
Sugarcane as feed: Proceeding of an FAO Expert Consultation held in
Santo Domingo. Dominican Republic, 7-11 July 1986. FAO Animal
production and Health paper No. 72. (Editors: Sansoucy, R., Aarst,
G. and Preston, T. R.) pp 164-169.
Green P S 1996. A Hawaiian Florilegium.
Gohl B 1981.Tropical feed information summaries and
nutritive values. FAO Anim. Prod. Health,
Rome.
Gohl B 1994. Tropical feeds 5.0 for widow. Revised by
Andrew Speedy, July 1994. Text, 1994, Food and Agriculture
Organization of the UN. Sofware (1994) Oxford Computer Juornals
Ltd.
Holness H D 1993.PIGS: The tropical Agriculturist: CTA (
the technical centre for Agricultural and Rural Co-operation.
(Editors: Rene coste and Anthony J Smith. pp 51-53
Holmes C W and Brookes I M 2001. What is high quality
pasture silage. dexcel farmfact, Massey University.
http://www.dexcel.co.nz/farmfacts.cfm?id=102
Hua-Wei Xu, Ji X M, He Z H, Shi W P, Zhu G H, Niu J K, Li B
S and Peng X X 2006. Oxalate accumulation and regulation is
independent of glycolate oxidase in rice leaves. Journal of
Experimental Botany 2006 57(9): 1899-1908; doi:10.1093/jxb/erj131
http://jxb.oxfordjournals.org/misc/terms.shtml
Khieu Borin 1996. A study on the use of sugar palm tree
(Borassus flabellifer) for different purposes in Cambodia.
(Msc Thesis in Sustainable Livestock Production, Swedish University
of Agricultural Sciences, Uppsala).
Lee W 1999. Taro (Colocasia esculenta), Southern
Illinois University Carbondale / Ethnobotanical Leaflets /.
http://www.siu.edu/~ebl/leaflets/taro.htm
Libert B and Franceschi V R 1987.
Oxalate in crop plants. J Agric Food Chem 35: 926-938.
http://pubs.acs.org/cgi-bin/abstract.cgi/jafcau/1987/35/i06/f-pdf/f_jf00078a019.pdf?sessid=6006l3
Lucas L 1982.
Plants of Old Hawaii.
Maclean M 1998. Livestock in Cambodian rice farming
system Cambodia-IRRI-Australia Project, Cambodia.
http://www.cardi.org.kh/Publication/publication13.htm
MAFF 2004. Statistic of Livestock. http://www.maff.gov.kh/en/statistics/livestock.html
McDonald P, Edwards R A, Greenhalgh J F D and Morgan C A
2002. Animal Nutrition. Sixth Edition. Longman Scientific and
Technical, Harlow, Essex, England.
Men B X, Ogle B and Preston T R 1995. Use of duckweed
(Lemna spp) as replacement for soya bean meal in a basal diet of
broken rice for fattening ducks. Livestock Research Rural
Development, 7(3): pp 5-8.
Mena A 1987. The utilization of sugar cane by-products as
substitutes for cereal in feeds animal feed: In: Proceeding of FAO
expert consultation on the substitution of imported concentrate
feeds in animal production systems in developing countries. FAO
animal production and health paper No. 63. (Editors: R Sansoucy, T
R Preston and R A Leng). pp 94-96.
Miller C D 1929.Food Values of Breadfruit, Taro Leaves,
Coconut and Sugar Cane. Bulletin 64, Bernice P. Bishop Museum,
Honolulu.
Miyasaka L S, Ogoshi R M, Tsuji G Y and Kodani L S 2003.
Site and planting date effect on Taro growth: comparison with aroid
model predictions. Agron. J. 95: 545-557.http://agron.scijournals.org/cgi/reprint/95/3/545.pdf
Naren T, Ogle B and Preston T R 1994. Optimum protein supply and level of inclusion of water spinach (Ipomoea aquatica) in sugar cane juice based diets for growing ducks. (MSc Thesis in sustainable livestock production, Swedish University Of Agricultural Sciences, Uppsala) pp 8-9.
Noonan S C and Savage G P 1999. Oxalate content of foods and its effect on humans. Asia Pacific Journal of Clinical Nutrition 8: (1): 64-74, 1999.
Ohio State University Extension 2001. Silage
Additives, Department of Horticulture and Crop Science, Columbus,
Ohio, USA. http://ohioline.osu.edu/agf-fact/0018.html
Oke O L 1969 .Oxalic acid in plants and in
nutrition. World Review of Nutrition and Dietetics, 10:
262-303
Paulas D and Muthukrishnan C R 1983. Products from palm
tree. FAO/DANIDA Palm tree workshop, Jaffna.
Phuc B H N, Ogle B and Preston T R 1994a. Effect of
protein supply in sugar cane juice based diet for growing pigs.
(Msc Thesis in Sustainable Livestock Production, Swedish University
of Agricultural Sciences, Uppsala). pp 4-10
Phuc B H N, Ogle B and Preston T R 1994b. Survey on the
use of sugar cane juice as energy source for growing pigs on small
farms in Vietnam. (Msc Thesis in Sustainable Livestock Production,
Swedish University of agricultural Sciences, Uppsala). pp
3-9
Phuc B H N 2000.tropical Forages for Growing pigs.
Digestion and Nutritive Value. Department of Animal Nutrition and
Management, Uppsala. pp 14
Preston T R 1991.
Sweet ration: Sugar cane as a
livestock feed. Ceres Rome. 23 (2): 39-40
Preston T R 2006. Forages as protein sources for pigs in the tropics. Workshop-seminar "Forages for Pigs and Rabbits" MEKARN-CelAgrid, Phnom Penh, Cambodia, 22-24 August, 2006. Article # 15. Retrieved February 8 (107) from http://www.mekarn.org/proprf/preston.htm
Rodríguez L, Peniche I and Preston T R
2006. Digestibility and nitrogen balance in growing pigs fed a
diet of sugar cane juice and fresh leaves of New Cocoyam
(Xanthosomasagittifolium) as partial or complete replacement
for soybean protein. Workshop-seminar "Forages for Pigs and
Rabbits" MEKARN-CelAgrid, Phnom Penh, Cambodia, 22-24 August, 2006.
Article # 15. Retrieved February 8 (107) from http://www.mekarn.org/proprf/rodr2
.htm
Rosales M, Preston T R and Vergas G E 1993.Advance in the
characterization of non-conversional resources with potential use
in animal nutrition. In: Anim. Prod. Developing countries (Eds).
Gill M., Over, E., Pollott, G. E. and Lawrence T. L. J. 16:
228-229.
Rosales M 1997. Trichantera gigantea (Humboldt and Bonpland) Nees: A review. Livestock Research Rural Development. 9 (3)l 9.http://www.cipav.org.co/lrrd/lrrd9/4/mauro942.htm
Sen Sovann 2002. Pig Production in Cambodia. In
'Priorities for Pig Research in Southeast Asia and the Pacific to
2010' pp. 22 - 28. Australian Centre for International Agricultural
Research, http://www.aciar.gov.au/web.nsf/doc/ACIA-5ND732
Canberra.
Speedy A W 1991. A comparison of sugar cane juice and maize as energy source in diet for pig with equal supply of amino acid. Livestock Research for Rural Development, 3(1). http://www.cipav.org.co/lrrd/lrrd3/1/speedy.htm
Standal B R 1983. Nutritive value. In JK Wang, ed,
Taro: A Review of Colocasia esculenta and Its Potentials.
University of Hawaii Press, Honolulu, pp 141-147
Tavana N G 2003: NTBG Internal Notes.
Tiep P S, Luc N V and Bien D H 2005: Processing and use
of Alocasia macrorrhiza (taro) roots for fattening pigs
under mountainous village conditions; Workshop-seminar
"Making better use of local feed resources" (Editors: Reg Preston
and Brian Ogle) MEKARN-CTU, Cantho, 23-25 May, 2005. Article #44.
Retrieved October 23 (106) from http://www.mekarn.org/proctu/tiep44.htm.
htm
Tiep P S, Luc N V, Tuyen T Q, Hung M N and Tran V T 2006.
Study on the use of Alocasiamacrorrhiza(roots and
leaves) in diets for crossbred growing pigs under mountainous
village conditions in northern Vietnam. Workshop-seminar "Forages
for Pigs and Rabbits" MEKARN-CelAgrid, Phnom Penh, Cambodia, 22-24
August, 2006. Article # 11. Retrieved February 8 (107) from
http://www.mekarn.org/proprf/tiep
.htm
Van Soest P J 1994. Nutritional ecology of the ruminant.
2nd edition. Cornell University Press, Ithaca,
USA.
Zindler-Frank E 1976. Oxalate
biosynthesis in relation to photosynthetic pathway and plant
productivity: a survey. Z Pflanzenphysiol 80: 1-13
[ISI]