Cassava hay production and its supplementation
effect on swamp buffaloes (Bubalus bubalis) in LOA
PDR; a review of the
literature
Ammaly Phengvilaysouk
Table of contents
Cassava production in Lao PDR..
……………………………………………………………………………..1
Nutritive values of cassava foliage
and root2
Cassava hay and root as a ruminant
feed. 3
Buffaloes production systems in Lao PDR.. 4
uffaloes management in lowland
areas. 5
Buffalo management in sloping land
zones. 5
The importance of buffaloes in
small-holder farming systems. 5
Supply of meat and milk.
Advantage of draft power and manure
Role of cassava hay supplementation for swamp buffaloes
Role of cassava hay supplementation for swamp buffaloes
Feed intake and digestibility of swamp buffaloes
Rumen ecology of swamp buffaloes
Cassava production in Lao PDR
The Lao PDR is a land-locked nation that lies within the
watershed of the Mekong River. Approximately 80% of the land
surfaces are hills and mountains. There are three main
agro-ecological zones consisting of uplands, plateau and lowlands.
The uplands are mountainous regions from 1,100 to 3,000 m altitude
(60% of the total land area). The plateau ranges from 800 to 1,300
m and the lowlands are plains less than 800 m above sea level
(Bouahom, 1995). The Lao PDR is seasonally tropical, with a
pronounced wet and dry season. The lowest levels of mean annual
rainfall are about 1,300 mm in the northwest, while the highest
level is 4,000 mm in the Annamite range in the south. The majority
of the lowlands have 1,500 to 2,000 mm annual rainfall (BCR, 2003).
Lao PDR has about 5 million hectares of land that is suitable
for cultivation. Of these only 800,000 hectares are planted with
rice or secondary food crops by farmers. Around 750,000 hectares
are used as pastureland and 50,000 hectares for ponds for
freshwater fish farming (MAF, 2002). In the uplands, complex soils,
which are strongly leached and acidic, predominate. Soils in the
lowlands are usually highly weathered, with moderate acid, and
classified texturally as loams, sandy loams and loamy sands. These
soils tend to be of low in organic matter content and have
therefore a low cation exchange capacity and low base saturation.
High levels of aluminium and iron are found in some soils, which
have a low water-holding capacity that makes them prone to drought.
Deficiencies of nitrogen and phosphorus are widespread. However,
cassava can grow in poor soil and can withstand drought. It is an
important reserve crop in countries with unreliable
rainfall.
Cassava is a plant that originated from South America and is
known under various names: Manihot esculenta, manioc, yucca
and tapioca, andbelongs to the family Euphorbiaceae. The
tubers are high in starch that can be used as animal feed and for
human consumption, while the leaves have high protein content. They
are important in many developing countries in Africa, South and
Central America, India and Southeast Asia (Food Safety, 2005). In
Laos, cassava is mostly grown on small farms, usually intercropped
with vegetables, sweet potatoes, melon, maize etc. In the
traditional cultivation, there are two common varieties of cassava;
the bitter and sweet. According to CIAT (2001) cassava is currently
the third most important crop in Laos, after rice and maize. It is
widely grown throughout the country by upland farmers but in small
areas using local varieties and with very low inputs. The roots are
used mainly for human consumption and for feeding livestock.
Recently, the use of cassava root and leaf has increased. In 2001,
researchers conducted experiments on cassava production at Houy
Khot station in Luang Prabang Province and the Livestock Research
Center (NamSuang) in Vientiane Province. Eight varieties were
introduced from Thailand and two local varieties were planted.
Cassava root yields were highest (20 to 25 tonnes per ha) for
varieties Rayong 72 and Rayong 90, and 13 to 15 tonnes per ha for
two local varieties at Houy Khot, while, cassava root yields were
highest (25 to 30 tonnes per ha) for varieties Rayong 72, Rayong 60
and KU50, versus 15 to 20 tonnes per ha for the two local varieties
at Namsuang (Vongsamphanh, 2003). In addition, several research
projects have been carried out on the use of cassava foliage to
feed animals: fresh foliage and DM yields ranged from 0.8 to 13.8,
and 0.3 to 4.0 tonnes per ha, respectively. The differences could
have been due to differences in planting space, study site and
subsequent harvestings (Chantaprasarn and Wanapat,
2005).
|
Table 1. Comparison of cassava planted area, harvested
area, production, and yield |
|||||
|
Year |
Country |
Planted Area
|
Harvested Area |
Production |
Yield |
|
|
|
(1000 ha) |
(1000 ha) |
(1000 metric tons) |
(kg/ha) |
|
1999 |
Laos |
13 |
13 |
81 |
6,176 |
|
2000 |
Laos |
19 |
19 |
118 |
6,057 |
|
2001 |
Laos |
16 |
16 |
101 |
6,174 |
|
2002 |
Laos |
4 |
4 |
29 |
7,080 |
|
2003 |
Laos |
20 |
20 |
150 |
7,687 |
|
2004 |
Laos |
8 |
8 |
56 |
6,810 |
|
2005 |
Laos |
7 |
7 |
51 |
7,580 |
|
Source: Office of
Agriculture Economics (2004) |
|||||
Nutritive values of cassava foliage and
root
Cassava roots are very starchy and therefore rich in
carbohydrates, a major source of energy. In fact, the cassava plant
is the highest producer of carbohydrates. It has been reported that
cassava can produce 250 x 103 calories per ha per day
compared to 176 x 103 for rice, 110 x 103 for
wheat, 200 x 103 for maize, and 114 x 103 for
sorghum, according to UNU (1980). The chemical composition of
cassava varies in different parts of the plant, different varieties
and it also depends on the location, age, stage of harvesting,
method of analysis, and environmental conditions. Although cassava
roots are rich in calories, they are grossly deficient in protein,
fat, and some minerals and vitamins. Consequently, cassava has
lower nutritional value than cereals, legumes, and even some other
roots and tuber crops, such as yams. According to UNU (1980) the
cassava root contains from 64 to 72% carbohydrates, which are
mostly starch, mainly amylose and amylopectin, and about 17% of
sucrose is found in sweet varieties, and small quantities of
fructose and dextrose. The lipid content of cassava is only 0.5 %.
Cassava roots are poor in proteins (1-2% CP), and the amino acid
profile of the cassava root is very low in some essential amino
acids, particularly lysine, methionine and tryptophan. However,
fermentation of the roots resulted in protein enrichment from 6 to
8%. Cassava is reasonably rich in calcium and vitamin C, but its
thiamine, riboflavin, and niacin contents are low. Generally,
cassava tubers are traditionally processed by a wide range of
methods, which reduce their toxicity, improve palatability and
convert the perishable fresh root into stable products; these
methods consist of different combinations of peeling, chopping,
grating, soaking, drying, boiling and fermenting (Tewe,
1991).
Cassava leaves are much richer in proteins than the roots.
Although the leaves contain far less methionine than the roots,
cassava-leaf has protein claimed to be superior to soybean protein.
Cassava leaves are used as a protein source when the roots are
harvested, but their intake and digestibility are low due to the
high level of condensed tannins (Reed et al., 1982; Onwuka,
1992). However, harvesting of cassava foliage at an early growth
stage (3 months) to make hay reduces the condensed tannin content
and increases the protein content to 25% of DM. Crude protein in
cassava hay has many essential amino acids similar to most
vegetable proteins and with very low hydrocyanic acid, as stated by
Wanapat et al. (1997). On the other hand, cassava leaves
contain on average 21% crude protein, but values can range from
16.7-39.9% (Ravindran, 1991). Khuong and Khang (2005) found that
fresh cassava foliage (DM basis) harvested at 45 days of re-growth
contains 20.1% DM, 21.5 CP,7.8 Ash, 28.8 ADF, 38.2 NDF and 3.5% CT
as compared to 20.2 CP, 36.4 ADF and 51.1% NDF in the experiment
reported by Khang and Wiktorsson (2004). This wide variability is
related to different cultivars, state of maturity, sampling
procedure, soil fertility and climate. Almost 85% of the crude
protein fraction is true protein (Eggum, 1970). The use of cassava
in livestock feeding has been limited, due to the fact that cassava
contains cyanogenic glucosides in the form of linamarin (93 per
cent), and lotaustralin (7 per cent). The amount of cyanogenic
glucosides varies with the part of the plant, age, variety and
environmental conditions such as soil, moisture, temperature, etc.
Certain varieties of cassava have long been designated as sweet or
bitter in relation to their cyanogenic glucoside content. The sweet
varieties are supposed to be much lower in HCN content than the
bitter varieties (Wanapat et al., 1997).
Cassava hay and root as a ruminant
feed
Cassava has one important characteristic, it can be managed to
maximize production of carbohydrate (in form of roots) or protein
by harvesting the leaves. For root production the growth cycle is
from 6 to 12 months, at the end of which the entire plant is
harvested. However, when maximum protein production is the aim, the
foliage is harvested at 2 to 3 month intervals by cutting the stems
at 50 to 70 cm above the ground, thereby encouraging the plant to
re-grow. Dual-purpose production systems are also possible whereby
one or two harvests of the leaves are taken before the plant is
allowed to continue the normal development of the roots, as
reported by Preston (2001). In addition, studies by Wanapat et
al. (1997; 2000a; 2001) revealed the potential uses of cassava
leaf and hay as a good source of protein. This was achieved by
collecting the leaf or whole crop at an early stage of growth, and
the harvesting of further biomass throughout the year. The
accumulated yield of cassava hay ranges from 2 to 8 tonnes of DM
per ha, depending on the variety, cultivation practice, use of
fertilizer and soil moisture (Wanapat, 2001a).
Cassava is an annual tropical tuber crop, which is nutritionally
valuable as a source of energy and protein for animals. Both fresh
and dried cassava roots are consumed in different forms by
ruminants (chopped, sliced, or ground). Dried cassava roots gave
satisfactory results as the principal energy source for dairy
cattle, intensive beef fattening and lamb growth. Cassava can
replace almost all of the grain in the diets with little reduction
in performance. Inclusion levels of up to 65%, preferably pelleted,
do not seem to affect animal health, carcass quality or overall
performance when the diets are carefully balanced. Palatability can
be enhanced by the addition of molasses if pelleting is not
possible (Animal Feed Resources Information System,
2006).
The protein content of cassava leaf and hay is relatively high,
while fiber levels are low. Moreover, levels of the essential amino
acids, methionine and threonine are similar to those found in
soybean meal. Cassava bushes three to four months old are harvested
by cutting about 40 cm above the ground and chopping in small
pieces by hand or in a stationary forage chopper to use as foliage
for cattle and other ruminants (Plant Guide, 2005). Cassava leaf or
hay contains 20 to 25 percent crude protein in the dry matter and
has minimal hydrocyanic acid (HCN) content, according to Wanapat
et al. (1997), who recommended the use of cassava hay
(leaves, stems and petiole) for feeding to ruminants. Wanapat et
al. (1989) reported that cassava hay, as supplement to rice
straw diets, increased rice straw intake, growth rates and
digestibility in cattle and buffaloes. Supplementation of cassava
hay to basal diets of untreated rice straw, cassava chips and
cassava residues increased benefits to farmers (Viet and Kien,
2005).
Cassava hay contains tannin-protein complexes, which can act as
a rumen by-pass protein and be digested in the small intestine.
Therefore, supplementation of cassava hay at a level of 1 to 2 kg
per head per day for ruminants could markedly reduce concentrate
requirements. The condensed tannins contained in cassava hay have
also a potential to reduce gastrointestinal nematodes; Kahn and
Diaz-Hernandez (2000) emphasized parasites as a serious problem for
ruminants in the tropics. Barry and Manley (1984) and Reed (1995)
found that condensed tannins in the feed at more than 6% of dry
matter reduce feed intake and digestibility; a CT level between 2
to 4% of DM helps to protect protein from rumen digestion and
thereby increases rumen by-pass protein. Condensed tannins and
protein can form a tannin-protein complex (TPC) by hydrogen
bonding, especially under alkaline conditions. TPC is stable at pH
3.5-7.0, but the complex will dissociate at pH<3.0 and pH>8.0
(Jones and Mangan, 1977). In addition, cassava hay is an excellent
multi-nutrient source for animals, especially because of its high
level of protein. Nutrient availability can be improved by grinding
and chopping. Overall, cassava has the potential to increase the
productivity and profitability of sustainable livestock production
systems in the tropics (Wanapat and Devendra, 1999; Wanapat et
al., 2000b; 2000c and Wanapat, 2001a).
|
Table
2. Chemical composition of cassava hay |
|||||||||
|
DM |
CP |
ash |
NDF |
ADF |
ADL |
CT |
Reference |
||
|
% |
--------------------- % DM
---------------------- |
|
|||||||
|
93.4 |
24.9 |
6.6 |
34.4 |
27.0 |
- |
3.8 |
Wanapat et al. (1997) |
||
|
92.0 |
23.5 |
4.2 |
55.2 |
31.4 |
- |
3.2 |
Netpana et al. (2001) |
||
|
92.0 |
22.0 |
7.1 |
58.8 |
32.0 |
- |
4.2 |
Poungchompu et al. (2001) |
||
|
92.3 |
23.4 |
13.5 |
50.4 |
45.0 |
- |
3.0 |
Wanapat et al. (2001) |
||
|
86.9 |
24.2 |
6.6 |
48.2 |
31.1 |
- |
1.8 |
Hong et al. (2003) |
||
|
92.3 |
20.6 |
7.5 |
55.0 |
38.9 |
16.8 |
3.3 |
Kiyothong and Wanapat (2003) |
||
|
93.7 |
27.3 |
8.0 |
67.7 |
41.7 |
13.2 |
3.6 |
Vongsamphanh and Wanapat (2004) |
||
|
94.6 |
23.2 |
6.6 |
41.0 |
25.5 |
7.8 |
3.4 |
Chantaprasarnand Wanapat (2005) |
||
|
92.3 |
20.6 |
6.6 |
49.7 |
32.1 |
10.8 |
3.5 |
Phengvilaysouk and Wanapat (2007) |
||
Buffalo production systems in Lao PDR
Swamp buffalo is indigenous in Lao PDR, and the majority
originated from stock domesticated within Lao PDR, nearby China and
Vietnam, and can be considered as an indigenous or traditional
breed (MAF, 2001). Most swamp buffaloes are completely grey in
color, and only a few are white. Normally, buffaloes have a lower
heat tolerance than cattle, are slate gray, droopy necked, and
ox-like with massive swept back horns. They wallow in any water or
mud pools they can find or make. The swamp buffalo which is
commonly seen in Laos is larger than native cattle, with males
reaching 450 kg and females 350 kg. Females tend to have their
first calf at four to five years, but calving intervals are lower
than for cattle. Some reports put the annual calving rate at less
than 50% (NAFRI et al., 2005). Commonly, buffalo is long
lived and female buffalo can produce healthy offspring with an age
above 20.
In Lao PDR, water buffaloes are predominantly of the swamp type,
raised in extensive low input systems that take advantage of
naturally occurring feed. The greatest difference is encountered
between predominantly lowland areas in the Mekong corridor zone and
the upland areas of the slopping land zones. Buffalo and cattle are
mostly found in the central region, where they graze on vacant
cropping areas for most of the year, but they also graze
extensively in the sloping lands zone (FAO, 2003). Devendra et
al. (1997) reported that the country is endowed with native
pasture grazing areas, and out of a total of 8.5 million ha, 2.4
are found in the low lands, 0.4 are found in the plateaus and 5.7
million ha are found in the upland areas,
respectively.
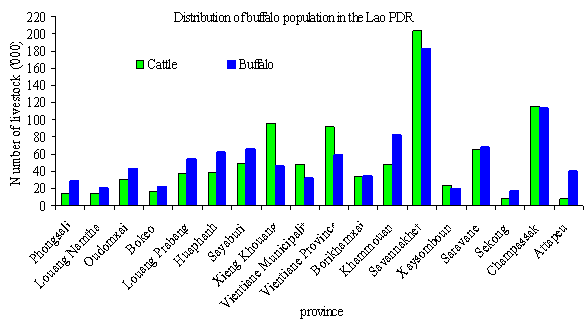
Figure 1. Number of large ruminants by
province in the Lao PDR ('000) [Sources: JICA-MAF
(2001), Agricultural Census (2000)
Buffalo management in lowland areas
Livestock is an important component of smallholder farms in
lowlands areas of Lao PDR. Sales of livestock account for more than
50% of cash income in many upland areas. Over 95% of livestock is
produced by smallholders (NAFRI et al., 2005). Buffalo
density is high in the central and southern regions where a higher
proportion of lowland rice is grown. This is the usual trend for
areas in Southeast Asia where rice is harvested only once a
year.
In these areas, buffalo are grazed on available cropping areas,
forest, communal lands, etc. In general, buffaloes depend mainly on
rice straw, stubbles, and re-growth of rice after harvest, and
grasses and weeds growing in and around fields. The management
systems are extensive, with animals being allowed to graze and mix
freely in large herds. During the rice growing season, animals
graze in the upland and the forest areas away from the rice fields
to avoid crop damage. They are also tied around the household and
fed with rice straw. The amount of available grazing and other
non-cropping areas are critical for animal production since there
are no other feed resources during this time of the year. Most
villages in the Mekong Corridor have access to such areas, which
are grazed heavily during the wet season. Management inputs are
minimal with animals grazing in small groups. When buffaloes are
needed to prepare land, they are kept near the rice fields (often
tethered) and are fed grass, rice bran and sometime sticky rice to
ensure that they are strong enough for land preparation.
In areas where irrigation enables farmers to grow two rice crops
per year, buffaloes can only graze the cropping areas for a few
months during the year. Therefore, they depend on other grazing
resources for most of the year. This limits the number of animals
that can be raised in these areas. Growing crops out of the normal
growing season also requires more herding and supervision of the
animals to avoid crop damage. More and more farmers invest in
hand-tractors for land preparation and transport in intensive
agricultural areas, and often sell their buffaloes to finance this
investment. Due to this buffalo numbers are declining in many
lowland areas (ILRI, 2002). On the other hand, the management of
health is minimal; some may be vaccinated against certain
infectious diseases, but most are not. Farmers have little interest
in bovine vaccination, except when disease outbreaks take place.
Common infectious diseases in bovines are foot-and-mouth disease of
type A, O, Asia-1, and Haemorrhagic Septicaemia is very serious in
buffalo. In addition, internal parasites such as Strongeloides and
Neoascaris are also found in buffalo (Chantalakhana and Skunmun,
2002).
Buffalo management
in sloping land zones
The sloping land zones include the upland and mountainous areas.
Buffaloes graze extensively in fallow upland fields, grazing areas
and forests. Management inputs are minimal for most of the year.
Buffalo and cattle production occurs mainly in mixed crop livestock
farming systems in the sloping lands zones rather than in the
Mekong Corridor. The reasons are feed resources limitation in the
lowland areas while in the sloping lands zone, more extensive
grazing is available, and the resources are ideally suited to breed
and supply buffaloes and cattle (ILRI, 2002).
In these areas, the traditional knowledge is different among the
ethnic groups and villagers; therefore the buffalo management
differs between areas. The Hmong are well known for their skills,
they build extensive fences using local materials to keep animals
from cropping areas during the wet season. Many villagers herd
buffalo communally in designated grazing areas. In other villages,
families manage animals individually. For one part of the year,
animals graze in remote areas where ample feed is available. At
other times, animals are brought back to pens every night. Farmers
in some villages provide additional feed to buffalo with newborn
calves and keep sick animals in special pens where they get
cut-and-carry feed. Most farmers provide salt to animals, often as
an incentive to return to the enclosure by themselves (NAFRI et
al., 2005)
The importance of buffaloes in small-holder
farming systems
Most Asian countries are agrarian, with 60-80% of the population
engaged in farm operations in one way or another. Livestock has
been an integral component of traditional agriculture for many
centuries in Asia (Nanda and Nakao, 2003). Livestock production is
firmly based in the smallholder farmers in both the Mekong Corridor
and the Sloping Lands. Nationally, about 30% of cash income from
agriculture was derived from livestock in 1997/98. In general, the
importance of livestock as a source of cash income is highest in
upland areas with poor access to markets where villages have to
carry goods over long distances to markets. This limits options to
crop with a high value per unit weight and livestock such as
buffaloes and cattle that can be walked to markets (ILRI, 2002).
Livestock provides many on-farm benefits; they are also a source of
savings to be sold when cash is needed. Only when households
accumulate enough livestock to feel financially secure are they
able to make long-term investments in their farming and livelihood
systems (e.g. sending children to high school, planting fruit
trees, buying a two-wheel tractor or micro rice mill), buffalo play
a pivotal role in the overall social development of small farmers.
It is the main source of draft power to cultivate crops and prepare
paddy rice fields, the main fertilizer and meat-milk supply for
small farmers in mountainous area (Tuyen and Ly,
2001)
Supply of meat and milk
Buffalo meat has a lower content of saturated fat than beef and
pork, and 40% less cholesterol, 55% less calories, 11% more protein
and 10% more minerals as compared to bovine meat, and therefore, it
is healthier. The quality has been markedly improved with the
crossbred in Australia, which it is hoped will be the future
standard in the 'Tender buff program' which has gained much
popularity in recent years (Lemcke, 1997). Buffalo milk is
healthier, because it is richer in saturated fatty acids. It is
high in total solids (18-23%), that make it useful to make into
cheese, butter fat, and several kinds of traditional sweets and ice
creams. Swamp buffalo milk has even higher fat (9-15%), protein
(7.1%), lactose (4.90%) and ash (0.89%) according to work by Thac
(1979). Riverine buffaloes produce more milk than swamp buffaloes.
The average lactation yield in the best known dairy buffalo breeds,
Murrah and Nili-ravi, is around 2000 L (Agarwal and Tomar, 1998).
Elite buffaloes with up to 6000 L also exist in India, Italy and
Pakistan; this indicates their great potential for milk production.
Working swamp buffalo produce 1.94 kg/day in Thailand (Khajarern
and Khajarern, 1990) and 1.55 kg/day in Vietnam (Thu, 1997) while a
non-working swamp buffalo may yield 2.15 kg/day (Gongzhen, 1996),
and crossed with Murrah can yield of 3.73 kg/day in 277 days lactation.
Advantage of draft power and
manure
Using buffalo for ploughing is definitely cost efficient in
rain-fed lowland mixed farming systems (Bunyavejchewin et
al., 1994). Based on profits produced over the investment, a
pair of buffalo is 2.6 times more efficient than a tractor (Thu
et al., 1995). Advantages include fuel savings, low
maintenance costs, high resale value, use of otherwise useless crop
residues, reduced dependence on external inputs and solidarity with
sustainability. Also, buffalo values increase over the years and
they need no costly fuel, maintenance, and they reproduce (FAO,
2000). Comparatively, machines require considerable monetary
investment and are usually much larger in size than required by a
peasant (Chantalakhana, 2001). Buffalo for work can start at
approximately 2 years of age or at 250 kg bodyweight. Young buffalo
can pull an approximately 50-80 kg plank or log in a plowed field
together with a trained buffalo. They are subjected to work for
approximately 1 h per day, gradually increasing to 3-4 h daily. The
average buffalo learns to obey commands within 3-4 weeks
(Saadullah, 1998). The power and efficiency of animals is increased
with age and gain in bodyweight. Buffaloes normally carry up to a
2.0 t load, but can pull more than six times their own bodyweight
continuously for 2-3 h, totaling 5-6 h daily in summer and 6-8 h in
winter (Acharya, 1988).The average buffalo works up to the age of
approximately 11 years. In Thailand, buffaloes work on average 5 h
per day for up to 146 days per year. Draft buffalo work
approximately 109 days/year in Vietnam (Sanh et al., 1995).
In China, one buffalo is sufficient to do all the work on 2-3
hectares of cultivated land. Swamp buffalo plow 0.30 ha/pair per
day and harrow 0.73 ha/pair per day (Thu et al., 1995).
Buffalo manure is an excellent source of nutrients for crops.
Approximately 40% of the value of an animal could be in the manure
it produces (Hoffmann, 1999). An adult buffalo produces 4-6 tonnes
of wet manure per year. Normally, dung is heaped to mature for a
few months and spread onto fields to maintain soil fertility.
Farmers in South-East Asia spread it in the early rainy season and
plow down before planting rice (Devendra and Pezo, 2002).
Role of cassava hay supplementation for swamp
buffaloes
In all countries of Southeast Asia, large ruminants are fed on
low protein diets and for a major part of the year are fed on crop
residues and mature grasses. These forages are often deficient in
crude protein and minerals. It should be possible to solve this
problem by supplementation of an extra protein source, particularly
cassava foliage. Cassava hay contains condensed tannins (CT) and
proanthocyanidins (PC), which are common in tropical plants. CT are
polyphenolics, which can easily be solubilized in water and can
precipitate protein. The presence of condensed tannins and protein
can result in the formation of tannin-protein complexes (TPC) by
hydrogen-bonding, especially under alkaline pH conditions. TPC
maintains its complex at pH 3.5-7.0, and will dissociate under pH
3.0 and over 8.0 (Jones and Mangan, 1977). Condensed tannins have
been found to increase N-recycling in the rumen and saliva (Reed,
1995). Moreover, they improve rumen ecology, especially through
enhancing microbial protein synthesis (Makkar et al., 1995;
McSweeney et al., 1999; Makkar, 2000).
In the absence of supplemental sources of bypass protein from
cassava leaf meals which have condensed tannins at optimal levels
to protect proteins from degradation in the rumen (Leng, 2005).
Wanapat (2001a) discovered that the condensed tannins (CT)
contained in cassava hay at levels of 3.05-4.20% are beneficial for
ruminants. Condensed tannins can protect proteins from fermentation
in the rumen and hence increase the supply of amino acids to the
small intestine (Kiyothong and Wanapat, 2003). Wanapat et
al. (1997) reported that condensed tannins in cassava hay form
a complex with the protein, so that much of the protein escapes the
rumen fermentation which contributes amino acids directly to the
animal.
However, in the tropical countries cassava is usually grown for
root harvesting, so cassava leaf is a residue from cassava root
production. Dried cassava leaf has successfully been used as
supplement for ruminants fed straw based diets and increased rice
straw intake according to Wanapat et al. (1992). This result
agrees with Vongsamphanh and Wanapat (2004) who summarized that
supplemented cassava hay increases both intake of rice straw and
total DMI, while Leng (1997) concluded that cassava hay
supplementation to native cattle, fed a rice straw basal diet and a
rumen supplement, improves feed intake, rumen ecology and
digestibility. Anexperiment was conducted on farm with supplemented
cassava hay (500 g/head/day), which increased the growth rate by
50% and improved the feed conversion by 16% in local beef cattle
fed on rice straw diets in the winter season in North Vietnam (Viet
and Kien, 2005).
Recently, attention is now being focused on the potential use of
whole cassava crop in integrated livestock production systems. In
Thailand, the cassava research programmes are mainly focused on
converting the cassava foliage into hay with the aim to reduce the
risks of HCN toxicity (Wanapat, 2001a). The results of several
experiments showed that cassava hay supplementation could replace
concentrate fed to dairy cows (Wanapat et al., 2000b), and
could reduce the level of nematode worm infestations in grazing
buffaloes (Neptana et al., 2001).Providing a good source of
roughage like cassava hay increases the ratio of protein to energy,
and hence increases productivity in ruminants.
Role of coconut oil supplementation for swamp
buffaloes
Dietary fats supply energy, carry fat-soluble vitamins (A, D, E,
K) and are a source of antioxidants and bioactive compounds. Fats
are also incorporated as structural components of the brain and
cell membranes. Coconut oil consists of highly saturated fat (over
90%) and is rich in lauric acid. Saturated fatty acids are more
digestible in ruminants than in nonruminants (Palmquist and
Jenkins, 1980). Scientific Psychic (2005) reported that coconut oil
is a common fatty acid which contains 8% unsaturated fatty acids 6%
oleic (C18:1), and 2% linoleic (C18:2) acid. Coconut oil also
contains 92% saturated fatty acids, including 6% capric (C10:0),
47% lauric (C12:0), 18% myristic (C14:0), 9% palmistic (C16:0), and
3% stearic (C18:0) acid.
Several studies were conducted to find out the appropriate utilization of oil, particularly supplementation to large ruminants. Wanapat et al. (2005) stated that energy in diets containing cassava chips and cassava hay are still low in energy density. Hence, it is possible to enhance energy density by other sources such as oil or fat. However, dietary fats are antimicrobial and interfere with normal function of the luminal microbes and result in fiber digestion depression. Fat is rapidly hydrolyzed in the rumen and the resultant long chain fatty acids are absorbed onto the fiber, which decreases its accessibility to microbial attack (Leng, 1987) or have a direct toxic effect on the luminal microorganisms, and hence reduce fiber digestibility. Depression of fiber digestion is most severe for fat sources which are high in unsaturated fatty acids than in saturated fatty acids (Jenkins, 1993).
Normally, fat content of ruminant diets is low (< 50 g/kg)
and if it is increased above 100 g/kg the activities of rumen
microbes are reduced (McDonald et al., 2002). Looper (2001);
Palmquist and Conrad (1978) suggested a limit of total fat from 6
to 7% in dry matter. While, Chantaprasarn (2005) indicated that
feeding fats to lactating dairy cows by using sunflower oil 2.5% in
the concentrate with cassava hay could be a way to meet the energy
and nutrient requirements to improve rumen ecology as well as milk
yield and quality. Wanapat et al. (2005) stated that
supplementation of coconut oil could improve rumen fermentation in
terms of the fermentation-end-products.
In contrast, coconut oil can be utilized as a dietary fat
supplement to eliminate protozoal population, its potential to
improve DM intake, digestibility and animal growth performance. In
addition, reduction of the protozoal population increased fungal
zoospores and bacteria (Leng, 1982; 1989); (Preston and Leng,
1987). Nguyen Thi Hong Nhan et al. (2001; 2005a); Mom Seng
et al. (2001) showed that oil eliminated protozoa from the
rumen and cattle grew faster. Nguyen Thi Hong Nhan et al.
(2005b) emphasized that drenching of soy bean oil at the rate of 6
ml/kg live weight at the start of the trial improved feed intake,
growth rate in cattle and economic profitability.
Feed intake and digestibility of swamp
buffaloes
Ruminants are adapted to utilize 'bullky' foods, such as hay and
straw. Rice straw and maize stover are roughages with a low
nutrient content and low digestibility, which are usually fed to
buffaloes as a main diet during the dry season in many Asian
countries (Thu, 2001). Therefore ruminants have evolved a special
system of digestion that involves microbial fermentation of feed
prior to its exposure to their own digestive enzymes. The success
of wild ruminants can be largely explained by their ability to
digest fibrous plant materials (Hungate, 1966). Ruminants
themselves do not produce fiber-degrading enzymes, but they harbor
bacteria, fungi, and protozoa that can. The host provides the
microorganisms with a suitable habitat for growth, and the microbes
supply the animal with protein, vitamins, and short-chain organic
acids. Microbial protein accounts for as much as 90% of the amino
acids reaching the small intestine, and energy from short-chain
organic acids (primarily acetic, propionic, and butyric acids).
Ruminal microorganisms can also ferment starch and sugars, and
these nonfibrous materials increase fermentation rate and animal
productivity (Nocek and Russell, 1988). The rumination and
fermentation are relatively slow processes, and fibrous feeds have
to stay in the digestive tract for along time until digestible
components are extracted. It is long recognized that there is a
positive relationship between the digestibility of feed and intake
in ruminants; total intake increases when digestibility is high,
but intake is actually more closely related to the rate of
digestion of diets than to digestibility; although the two last
measures are generally related to one another. In other words, feed
that are rapidly digested, and have a high digestibility, promote
high intake. The faster the rate of digestion, the more rapidly is
the digestive tract emptied, and the more space is made available
for the next meal. The primary chemical component of feeds that
determines their rate of digestion is neutral-detergent fibrous
(NDF), which is itself a measure of cell-wall content, the physical
form of cell walls also effect intake. The mechanical grinding of
roughages partially destroys the structural organization of cell
walls, thereby accelerating their breakdown in the rumen and
increasing feed intake.
However, nutrient deficiencies that reduce the activities of
rumen microorganisms are liable to reduce feed intake. The most
common are proteins and nitrogen deficiency, which can correct by
supplementation with rumen-degradable protein or with simple source
of nitrogen such as urea. The effects of ammonia availability
through digestibility and straw intake of cattle; there are two
critical ammonia levels. These are 80-100 mg N/l for optimum
fibrolytic activity and 150-250 mg N/l for optimum microbial
activity (McDonald et al., 2002). In the present study with
animals fed on low quality rice straw there were significant
improvements of DM, OM and NDF diet digestibility for the
supplemented diets compared to the non-supplemented diet. Murphy
(1990) reported that increases in nitrogen supply or sparing
effects of the branched amino acids led to an increase in the
numbers of cellulolytic bacteria and fiber digestion. This agrees
with Yuangklang et al. (2001), who found that DM intake and
digestibility coefficients were higher for urea-treated rice straw
and for cassava hay than for rice straw and ruzi grass hay.
Supplements of undegradable protein may also increase the intake of
low-protein forages. Other nutrient deficiencies that are liable to
restrict feed intake in ruminants are sulphur, phosphorus, sodium
and cobalt.
Animal factors affect the intake in ruminants, and if capacity of the rumen is a critical factor in determining the feed intake of ruminants, then circumstances that change the relationship between the size of the rumen and the size of the whole animal are likely to affect intake. Thus, any variations betwe